Imaging the Centromedian Thalamic Nucleus Using Quantitative Susceptibility Mapping
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

MRI Atlas of the Human Deep Brain Jean-Jacques Lemaire
MRI Atlas of the Human Deep Brain Jean-Jacques Lemaire To cite this version: Jean-Jacques Lemaire. MRI Atlas of the Human Deep Brain. 2019. hal-02116633 HAL Id: hal-02116633 https://hal.uca.fr/hal-02116633 Preprint submitted on 1 May 2019 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Distributed under a Creative Commons Attribution - NonCommercial - NoDerivatives| 4.0 International License MRI ATLAS of the HUMAN DEEP BRAIN Jean-Jacques Lemaire, MD, PhD, neurosurgeon, University Hospital of Clermont-Ferrand, Université Clermont Auvergne, CNRS, SIGMA, France This work is licensed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/ or send a letter to Creative Commons, PO Box 1866, Mountain View, CA 94042, USA. Terminologia Foundational Model Terminologia MRI Deep Brain Atlas NeuroNames (ID) neuroanatomica usages, classical and french terminologies of Anatomy (ID) Anatomica 1998 (ID) 2017 http://fipat.library.dal.ca In -

Magnetic Resonance Imaging of Mediodorsal, Pulvinar, and Centromedian Nuclei of the Thalamus in Patients with Schizophrenia
ORIGINAL ARTICLE Magnetic Resonance Imaging of Mediodorsal, Pulvinar, and Centromedian Nuclei of the Thalamus in Patients With Schizophrenia Eileen M. Kemether, MD; Monte S. Buchsbaum, MD; William Byne, MD, PhD; Erin A. Hazlett, PhD; Mehmet Haznedar, MD; Adam M. Brickman, MPhil; Jimcy Platholi, MA; Rachel Bloom Background: Postmortem and magnetic resonance im- reduced in all 3 nuclei; differences in relative reduction aging (MRI) data have suggested volume reductions in did not differ among the nuclei. The remainder of the the mediodorsal (MDN) and pulvinar nuclei (PUL) of the thalamic volume (whole thalamus minus the volume of thalamus. The centromedian nucleus (CMN), impor- the 3 delineated nuclei) was not different between schizo- tant in attention and arousal, has not been previously stud- phrenic patients and controls, indicating that the vol- ied with MRI. ume reduction was specific to these nuclei. Volume rela- tive to brain size was reduced in all 3 nuclei and remained Methods: A sample of 41 patients with schizophrenia significant when only patients who had never been ex- (32 men and 9 women) and 60 healthy volunteers (45 posed to neuroleptic medication (n=15) were consid- men and 15 women) underwent assessment with high- ered. For the MDN, women had larger relative volumes resolution 1.2-mm thick anatomical MRI. Images were than men among controls, but men had larger volumes differentiated to enhance the edges and outline of the than women among schizophrenic patients. whole thalamus, and the MDN, PUL, and CMN were out- lined on all slices by a tracer masked to diagnostic Conclusions: Three association regions of the thala- status. -

Targeting the Centromedian Thalamic Nucleus for Deep Brain Stimulation
medRxiv preprint doi: https://doi.org/10.1101/19008136; this version posted October 18, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted medRxiv a license to display the preprint in perpetuity. It is made available under a CC-BY-NC-ND 4.0 International license . Title: Targeting the centromedian thalamic nucleus for deep brain stimulation Authors: Aaron E.L Warren1,2,3, Linda J. Dalic1,3,4, Wesley Thevathasan4,5,6, Annie Roten1, Kristian J. Bulluss5,7,8, John S. Archer1,2,3,4 1Department of Medicine (Austin Health), University of Melbourne, Heidelberg, Victoria, Australia 2Murdoch Children’s Research Institute, Parkville, Victoria, Australia 3The Florey Institute of Neuroscience and Mental Health, Heidelberg, Victoria, Australia 4Department of Neurology, Austin Health, Heidelberg, Victoria, Australia 5Bionics Institute, East Melbourne, Victoria, Australia 6Department of Medicine, University of Melbourne, and Department of Neurology, The Royal Melbourne Hospital, Parkville, Victoria, Australia 7Department of Neurosurgery, Austin Health, Heidelberg, Victoria, Australia 8Department of Surgery, University of Melbourne, Parkville, Victoria, Australia Corresponding author: Aaron E.L. Warren Ph: +613 9035 7110 Email: [email protected] Address: Melbourne Brain Centre, 245 Burgundy Street, Heidelberg, VIC, Australia 3084 Key words: Deep brain stimulation, centromedian nucleus, thalamus, epilepsy, Lennox- Gastaut syndrome, microelectrode recording, fMRI 1 NOTE: This preprint reports new research that has not been certified by peer review and should not be used to guide clinical practice. medRxiv preprint doi: https://doi.org/10.1101/19008136; this version posted October 18, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted medRxiv a license to display the preprint in perpetuity. -

Thalamic Projection to Frontal Cortex in Man*
J Neurol Neurosurg Psychiatry: first published as 10.1136/jnnp.13.3.198 on 1 August 1950. Downloaded from J. Neurol. Neurosurg. Psychiat., 1950, 13, 198. THALAMIC PROJECTION TO FRONTAL CORTEX IN MAN* BY TURNER McLARDY From the Department of Neuropathology, the Institute ofPsychiatry, University of London The object of this communication is to report the ferential organization such that adjacent regions in present position at the Maudsley laboratory of the nucleus project to adjacent areas in the cortex. research on the projection of nerve cells of the The nucleus submedius, when present, projects human thalamus on to frontal cortex. Previous, to the same cortical field as the adjacent part of the more tentative, reports on this topic were published dorsomedial nucleus, namely area 8. by Meyer, Beck, and McLardy in 1947 and by The other intralaminar nuclei, including the Meyer, McLardy, and Beck in 1948. centromedian nucleus and the parafascicular nucleus, The main material used consisted of 54 cerebral and the midline nuclei, have no projection to the hemispheres with varied leucotomy lesions. A few frontal cortex, so far as can be argued from observa- cases oftraumatic and of vascular damage to frontal tions on retrograde degeneration after fairly circum- cortex were also employed. Cortical areas involved scribed damage to the frontal lobe. There is, in the primary lesions were correlated with the however, some indication that some of them may position of retrograde nerve cell degeneration and possess a diffuse cortical projection. glial proliferation observed in Nissl-stained serial The agranular area 6 receives projections from sections of the thalamus. -

Habenula Deep Brain Stimulation for Intractable Schizophrenia: a Pilot Study
NEUROSURGICAL FOCUS Neurosurg Focus 49 (1):E9, 2020 Habenula deep brain stimulation for intractable schizophrenia: a pilot study Yuhan Wang, MS,1 Chencheng Zhang, MD, PhD,1 Yingying Zhang, MSc,1 Hengfen Gong, MD,2 Jun Li, PhD,1 Haiyan Jin, MD,3 Dianyou Li, MD, PhD,1 Dengtang Liu, MD, PhD,4 and Bomin Sun, MD, PhD1 1Department of Functional Neurosurgery, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine; 2Department of Psychiatry, Pudong Mental Health Center, Shanghai; 3Department of Psychiatry, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine; and 4First-episode Schizophrenia and Early Psychosis Program, Division of Psychotic Disorders, Shanghai Mental Health Center, Shanghai Jiao Tong University School of Medicine, Shanghai, China Schizophrenia is a psychiatric disorder associated with significant morbidity and mortality. Although antipsychotic medications and electroconvulsive therapy can be used to manage the clinical symptoms of schizophrenia, a substantial portion (10%–30%) of patients do not clinically respond to these treatments or cannot tolerate the side effects. Recently, deep brain stimulation (DBS) has emerged as a promising safe and effective therapeutic intervention for various psychi- atric disorders. Here, the authors explore the utility of DBS of the habenula (HB) in the clinical management of 2 young adult male patients with severe, chronic, and treatment-resistant schizophrenia. After HB DBS surgery, both patients experienced improvements in clinical symptoms during the first 6 months of treatment. However, only 1 patient retained the clinical benefits and reached a favorable outcome at 12-month follow-up. The symptoms of the other patient sub- sequently worsened and became so profound that he needed to be hospitalized at 10-month follow-up and withdrawn from further study participation. -

Modulation of Fibers to Motor Cortex During Thalamic DBS in Tourette Patients Correlates with Tic Reduction
brain sciences Article Modulation of Fibers to Motor Cortex during Thalamic DBS in Tourette Patients Correlates with Tic Reduction Pablo Andrade 1,* , Petra Heiden 1,2, Moritz Hoevels 1, Marc Schlamann 3, Juan C. Baldermann 4,5, Daniel Huys 4 and Veerle Visser-Vandewalle 1 1 Department of Stereotactic and Functional Neurosurgery, University Hospital of Cologne, 50397 Cologne, Germany; [email protected] (P.H.); [email protected] (M.H.); [email protected] (V.V.-V.) 2 Department of Neurosurgery, University Hospital of Cologne, 50397 Cologne, Germany 3 Department of Neuroradiology, University Hospital of Cologne, 50397 Cologne, Germany; [email protected] 4 Department of Psychiatry and Psychotherapy, University Hospital of Cologne, 50397 Cologne, Germany; [email protected] (J.C.B.); [email protected] (D.H.) 5 Department of Neurology, University Hospital of Cologne, 50397 Cologne, Germany * Correspondence: [email protected]; Tel.: +49-221-478-82737; Fax: +49-221-478-82824 Received: 12 April 2020; Accepted: 11 May 2020; Published: 15 May 2020 Abstract: Probabilistic tractography in Tourette syndrome (TS) patients have shown an alteration in the connectivity of the primary motor cortex and supplementary motor area with the striatum and thalamus, suggesting an abnormal connectivity of the cortico-striatum-thalamocortical-pathways in TS. Deep brain stimulation (DBS) of the centromedian nucleus–nucleus ventrooralis internus (CM-Voi complex) in the thalamus is an effective treatment for refractory TS patients. We investigated the connectivity of activated fibers from CM-Voi to the motor cortex and its correlation between these projections and their clinical outcome. -

Thalamic Interaction Between the Input and the Output Systems of the Basal Ganglia
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Dadun, University of Navarra Journal of Chemical Neuroanatomy 16 (1999) 187–200 Review Thalamic interaction between the input and the output systems of the basal ganglia Elisa Mengual a, Silvano de las Heras b, Elena Erro a, Jose´ Luis Lanciego a, Jose´ Manuel Gime´nez-Amaya a,* a Departamento de Anatomı´a, Facultad de Medicina, Uni6ersidad de Na6arra, C/ Irunlarrea, 1, 31008 Pamplona, Spain b Departamento de Morfologı´a, Facultad de Medicina, Uni6ersidad Auto´noma de Madrid, 28029 Madrid, Spain Received 6 April 1998; received in revised form 22 October 1998; accepted 14 February 1999 Abstract The striatal return through the thalamus is largely neglected in current studies dealing with basal ganglia function, and its role within this circuitry remains obscure. In this contribution the thalamus is regarded as an important place of interaction between the input and the output organization of the basal ganglia. In support of this idea, a brief overview is provided of some of the most recent findings concerning the thalamus in relation to the basal ganglia circuitry. In particular, we have focused on the thalamostriatal projections themselves, on the output of the basal ganglia to the thalamus and also on the overlapping territories between the thalamic projection of the output nuclei and the thalamostriatal neurons. These data support the existence of several thalamic feedback circuits within the basal ganglia neural system. Finally, some considerations are provided upon the functional significance of these thalamic feedback circuits in the overall organization of the basal ganglia. -
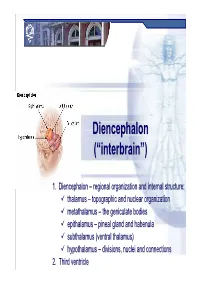
Diencephalondiencephalon ((““Interbraininterbrain ””))
DiencephalonDiencephalon ((““interbraininterbrain ””)) 1. Diencephalon – regional organization and internal structure: thalamus – topographic and nuclear organization metathalamus – the geniculate bodies epithalamus – pineal gland and habenula subthalamus (ventral thalamus) hypothalamus – divisions, nuclei and connections 2. Third ventricle Diencephalon EmbryologicEmbryologic developmentdevelopment Embryonic origin:origin side walls of the prosencephalon (forebrain) Location – at the midline of the brain: caudally – mesencephalon cranially – telencephalon Functions: relay system between sensory input neurons and other parts of the brain works in tandem with the limbic system Prof. Dr. Nikolai Lazarov 2 Diencephalon DiencephalonDiencephalon –– grossgross structurestructure andand partsparts Prof. Dr. Nikolai Lazarov 3 Thalamus ThalamusThalamus –– externalexternal featuresfeatures two egg -shaped lobes of grey matter Gr. θάλαµος = room , chamber third ventricle medially Gr. θάλαµος = room , chamber hypothalamus hypothalamic sulcus Thalamus dorsalis : nuclear complex – 2% of the total brain rostral pole = tuberculum anterius nuclear complex – 2% of the total brain thalami about 80% caudal pole = pulvinar thalami of diencephalic mass (“cushioned seat ”) ~30 mm long interthalamic adhesion ~20 mm wide lamina affixa ~20 mm tall stria terminalis thalami Prof. Dr. Nikolai Lazarov 4 Thalamus ThalamusThalamus –– internalinternal structurestructure internal medullary lamina three major nuclear masses: (medial) – Y-shaped -

Functioning of Circuits Connecting Thalamus PMU ?> and Cortex
Functioning of Circuits Connecting Thalamus and Cortex S. Murray Sherman*1 ABSTRACT Glutamatergic pathways in thalamus and cortex are divided into two distinct classes: driver, which carries the main information between cells, and modulator, which modifies how driver inputs func- tion. Identifying driver inputs helps to reveal functional computational circuits, and one set of such circuits identified by this approach are cortico-thalamo-cortical (or transthalamic corticocortical) circuits. This, in turn, leads to the conclusion that there are two types of thalamic relay: first order nuclei (such as the lateral geniculate nucleus) that relay driver input from a subcortical source (i.e., retina), and higher order nuclei (such as the pulvinar) which are involved in these transthalamic pathways by relaying driver input from layer 5 of one cortical area to another. This thalamic division is also seen in other sensory pathways and beyond these so that most of thalamus by volume consists of higher-order relays. Many, and perhaps all, direct driver connections between cortical areas are paralleled by an indirect cortico-thalamo-cortical (transthalamic) driver route involving higher order thalamic relays. Such thalamic relays represent a heretofore unappreci- ated role in cortical functioning, and this assessment challenges and extends conventional views regarding both the role of thalamus and mechanisms of corticocortical communication. Finally, many and perhaps the vast majority of driver inputs relayed through thalamus arrive via branching axons, with extrathalamic targets often being subcortical motor centers. This raises the possibility that inputs relayed by thalamus to cortex also serve as efference copies, and this may represent an important feature of information relayed up the cortical hierarchy via transthalamic circuits. -
Vestibular Interactions in the Thalamus
MINI REVIEW published: 02 December 2015 doi: 10.3389/fncir.2015.00079 Vestibular Interactions in the Thalamus Rajiv Wijesinghe 1, Dario A. Protti 2 and Aaron J. Camp 1* 1 Sensory Systems and Integration Laboratory, Sydney Medical School, Discipline of Biomedical Science, University of Sydney, Sydney, NSW, Australia, 2 Vision Laboratory, Sydney Medical School, Discipline of Physiology, University of Sydney, Sydney, NSW, Australia It has long been known that the vast majority of all information en route to the cerebral cortex must first pass through the thalamus. The long held view that the thalamus serves as a simple hi fidelity relay station for sensory information to the cortex, however, has over recent years been dispelled. Indeed, multiple projections from the vestibular nuclei to thalamic nuclei (including the ventrobasal nuclei, and the geniculate bodies)- regions typically associated with other modalities- have been described. Further, some thalamic neurons have been shown to respond to stimuli presented from across sensory modalities. For example, neurons in the rat anterodorsal and laterodorsal nuclei of the thalamus respond to visual, vestibular, proprioceptive and somatosensory stimuli and integrate this information to compute heading within the environment. Together, these findings imply that the thalamus serves crucial integrative functions, at least in regard to vestibular processing, beyond that imparted by a “simple” relay. In this mini review we outline the vestibular inputs to the thalamus and provide some clinical context for vestibular interactions in the thalamus. We then focus on how vestibular inputs interact with other sensory systems and discuss the multisensory integration properties of the thalamus. Edited by: W. -
The Interpretation Ofthe Degenerative Changes In
J Neurol Neurosurg Psychiatry: first published as 10.1136/jnnp.30.2.140 on 1 April 1967. Downloaded from J. Neurol. Neurosurg. Psychiat., 1967, 30, 140 The interpretation of the degenerative changes in the intralaminar nuclei of the thalamus T. P. S. POWELL AND W. M. COWAN From the University of Oxford, Department of Human Anatomy, South Parks Road, Oxford Although the intralaminar nuclei of the thalamus rostral part of the corpus callosum. In three brains are now known to have an organized, extrathalamic stereotactic lesions had been placed in the thalamus for projection through the internal capsule (Droogleever another investigation, and the remaining brains had and lesions in various parts of the cerebral cortex. All the Fortuyn, and Stefens, 1951; Nashold, Hanbery, animals with striatal lesions and four with cortical Olszewski, 1955; Powell and Cowan, 1956), the ablations were allowed to survive for periods ranging precise mode of termination of the efferents from between four and 18 weeks. These brains were fixed either these nuclei remains problematical. The experimental in 70% alcohol and 2% acetic acid or in 10% formol- evidence bearing upon the projection of these nuclei saline, and a regular one in five or one in 10 series of which is available at present permits of two possible sections throughout most of thc cerebral hemisphere was interpretations. Because the cells of these nuclei mounted and stained with thionine. The remainingProtected by copyright. undergo a certain degree of cellular degeneration as animals with cortical lesions survived for between one the result of large lesions of the cerebral cortex it and two weeks, were perfused with 10% formol-saline, that the intralaminar nuclei send and after a further period of fixation were sectioned on a has been suggested freezing microtome and stained according to the method their axons, or axon collaterals, directly to the of Nauta (1957). -

Willim R. Kcnler Supported. By: NASA Task No. 5202-06-~
Nucleus ol'@uys -c_- Willim R. Kcnler 3ieuro-Dioiogy 3ranc11 Office of Life Sclences A.zes 3esearcii Center - . I N U ITHRUI .. L.. ea (CODE1 Supported. by: NASA Task No. 5202-06-~ GPO PRICE !§ CFSTI PRICE(S) $ t Hard copy (HC) *w Microfiche (MF) *sQ? ff 653 July 65 W. R. Mehler __l' ---_cI- "Tee number ofbhalamic nucl5)has increased in the last I"ew years. To the four classical centers that were described by Burdach one shbuld add the Centre median of Luys and the semi-lunar of Flechsig. Finally, von Monakow and Nissl have subdivided each pincipl nucleus into a large number of secondary Pleiades on me basis of the relative volume 02 cells and the number of mye- l-zeted fibers of the inzerstltial plexus. It is our belief tket i.ic individuality of ti;e na2arnic cenic;s should not -02 based uaon the aspect of such centers'c; TY.NiTs1 or-Weigert sections, but upon ?;'ne study of their fine+nato-$,as it appears in silver chromate smlnecl scctions acd, above all, upon tke specificity of their ~o:~~.~ctLons.A nucleus shodd only be considered as an individual cLLAUerwrien besides having peculiar stxc-cural features, its h;i'erent and efferent fibers are spec:fic .'I Santiago Raymori y Caial l9Oi lkth International Medical Congress Translation by Enrique Ramon-Moliner ._ 1;: 1931 3ioch added a "note" to his then definitive studies on the cii.rAiCc._,:idlonor' the Carnivora (Rioch,l929 a,b, 1931a). This I addendum 1 at-cer;rptzd to rectify his earlier incluslon of the nucleus centre median , of LL.J, In ;Le region 'he -identifie$-as tne nucleus parafascicularis.