Armine Abrahamyan Distribution Modeling and Population Ecology Of
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Local Level Risk Management M a N U
LOCAL LEVEL RISK MANAGEMENT M A N U A L Y E R E V A N 2012 1 LLRM EXECUTIVE LOCAL LEVEL RISK IMPLEMENTATION BACKGROUND 2 3 SUMMARY MANAGEMENT (LLRM) / FORMAT EXPERIENCE IN ARMENIA VULNERABILITY AND GENERAL APPROACHES AND CAPACITY 1.1 INFORMATION 2.1 3.1 PRINCIPLES APPLIED ASSESSMENT (VCA) HAZARDS RESOURCES AND THREATENING 3.2 PRACTICAL CASES TOOLS ARMENIA PROCESS A PREPARATORY PHASE DATA COLLECTION B AND RESEARCH C ANALYSIS D TOOL KIT PLANNING DRR MAINSTREAMING INTO DEVELOPMENT PLANS / DESCRIPTION AND PLANNING TOOLS IMPLEMENTATION, MONITORING AND EVALUATION DRR AND CLIMATE LLRM RISK MANAGEMENT DRR AND GENDER M A N U A L EQUITY 2 Authors: Ashot Sargsyan UNDP, DRR Adviser Armen Chilingaryan UNDP, DRR Project Coordinator Susanna Mnatsakanyan UNDP DRR Project VCA Expert Experts: Hamlet Matevosyan Rector of the Crisis Management State Academy of the Ministry of Emergency Situations Hasmik Saroyan Climate Risk Management Expert LLRM/VCA implementation Armen Arakelyan Specialist Head of “Lore” Rescue Team This manual is prepared and published with financial support from UNDP within the framework of the Project Strengthening of National Disaster Preparedness and Risk Reduction Capacities in Armenia. Empowered lives The views expressed in the publication are those of the author(s) and do not necessarily represent those of the Resilient nations United Nations or UNDP. 3 ACKNOWLEDGEMENTS This manual is a result of consolidation of collective efforts of many professionals and experts from different organizations and agencies – members of the UN extended Disaster Management Team, which worked during the years hand-to-hand to support and facilitate the strengthening of Disaster Management national system in Armenia. -

Agricultural Value-Chains Assessment Report April 2020.Pdf
1 2 ABOUT THE EUROPEAN UNION The Member States of the European Union have decided to link together their know-how, resources and destinies. Together, they have built a zone of stability, democracy and sustainable development whilst maintaining cultural diversity, tolerance and individual freedoms. The European Union is committed to sharing its achievements and its values with countries and peoples beyond its borders. ABOUT THE PUBLICATION: This publication was produced within the framework of the EU Green Agriculture Initiative in Armenia (EU-GAIA) project, which is funded by the European Union (EU) and the Austrian Development Cooperation (ADC), and implemented by the Austrian Development Agency (ADA) and the United Nations Development Programme (UNDP) in Armenia. In the framework of the European Union-funded EU-GAIA project, the Austrian Development Agency (ADA) hereby agrees that the reader uses this manual solely for non-commercial purposes. Prepared by: EV Consulting CJSC © 2020 Austrian Development Agency. All rights reserved. Licensed to the European Union under conditions. Yerevan, 2020 3 CONTENTS LIST OF ABBREVIATIONS ................................................................................................................................ 5 1. INTRODUCTION AND BACKGROUND ..................................................................................................... 6 2. OVERVIEW OF DEVELOPMENT DYNAMICS OF AGRICULTURE IN ARMENIA AND GOVERNMENT PRIORITIES..................................................................................................................................................... -

Republic of Armenia Infrastructure and Rural Finance Support Programme Final Project Design Report Main Report
Republic of Armenia Infrastructure and Rural Finance Support Programme Final Project Design Report Main report and annexes Document Date: May 2014 Project No. 1690 Report No: ____-AM Near East, North Africa and Europe Division Programme Management Department Republic of Armenia Infrastructure and Rural Finance Support Programme Final project design report Main report Contents Page Currency equivalents iii Abbreviations and acronyms iii Map of IFAD Operations in the Country v Map of the Programme area vi Executive Summary vii Logical Framework xiii I. STRATEGIC CONTEXT AND RATIONALE 1 A. Background 1 B. Country and Rural Development Context 2 C. Rationale 6 II. PROGRAMME DESCRIPTION 9 A. Development Objectives 9 B. Programme Components 9 C. Target Group and Programme Area 10 D. Description of Inputs and Outputs/Outcomes 12 III. PROGRAMME IMPLEMENTATION 17 A. Approaches 17 B. Implementation Schedule for Civil Works 20 C. Planning, M&E, Learning and Knowledge Management 23 D. Financial Management 24 E. Procurement and Governance 26 F. Supervision 28 G. Risk Identification and Mitigation 29 IV. PROGRAMME COSTS, FINANCING, BENEFITS AND SUSTAINABILITY 32 A. Estimated Programme Costs 32 B. Proposed Programme Financing 33 C. Summary Benefits and Economic Analysis 34 D. Sustainability 38 TABLES Table 1: Armenia: Reduction in the Incidence of Poverty 1998/99 to 2007 4 Table 2: Programme Costs by Component 32 Table 3: Proposed Programme Financing Sources by Type of Investment 33 Table 4: Proposed Programme Financing Sources by Component 33 Table -

Years in Armenia
1O Years of Independence and Transition in Armenia National Human Development Report Armenia 2OO1 Team of Authors National Project Director Zorab Mnatsakanyan National Project Coordinator-Consultant Nune Yeghiazaryan Chapter 1 Mkrtich Zardaryan, PhD (History) Aram Harutunyan Khachatur Bezirchyan, PhD (Biology) Avetik Ishkhanyan, PhD (Geology) Boris Navasardyan Ashot Zalinyan, PhD (Economics) Sos Gimishyan Edward Ordyan, Doctor of Science (Economics) Chapter 2 Ara Karyan, PhD (Economics) Stepan Mantarlyan, PhD (Economics) Bagrat Tunyan, PhD (Economics) Narine Sahakyan, PhD (Economics) Chapter 3 Gyulnara Hovhanessyan, PhD (Economics) Anahit Sargsyan, PhD (Economics) "Spiritual Armenia" NGO, Anahit Harutunyan, PhD (Philology) Chapter 4 Viktoria Ter-Nikoghosyan, PhD (Biophysics) Aghavni Karakhanyan Economic Research Institute of the RA Ministry of Finance & Economy, Armenak Darbinyan, PhD (Economics) Nune Yeghiazaryan Hrach Galstyan, PhD (Biology) Authors of Boxes Information System of St. Echmiadzin Sergey Vardanyan, "Spiritual Armenia" NGO Gagik Gyurjyan, Head of RA Department of Preservation of Historical and Cultural Monuments Gevorg Poghosyan, Armenian Sociological Association Bagrat Sahakyan Yerevan Press Club "Logika", Independent Research Center on Business and Finance Arevik Petrosian, Aharon Mkrtchian, Public Sector Reform Commission, Working Group on Civil Service Reforms Armen Khudaverdian, Secretary of Public Sector Reform Commission "Orran" Benevolent NGO IOM/Armenia office Karine Danielian, Association "For Sustainable Human -

Stocktaking Exercise to Identify Legal, Institutional, Vulnerability Assessment and Adaptation Gaps and Barriers in Water Resour
“National Adaptation Plan to advance medium and long-term adaptation planning in Armenia” UNDP-GCF Project Stocktaking exercise to identify legal, institutional, vulnerability assessment and adaptation gaps and barriers in water resources management under climate change conditions Prepared by “Geoinfo” LLC Contract Number: RFP 088/2019 YEREVAN 2020 Produced by GeoInfo, Ltd., Charents 1, Yerevan, Armenia Action coordinated by Vahagn Tonoyan Date 11.06.2020 Version Final Produced for UNDP Climate Change Program Financed by: GCF-UNDP “National Adaptation Plan to advance medium and long-term adaptation planning in Armenia” project Authors National experts: Liana Margaryan, Aleksandr Arakelyan, Edgar Misakyan, Olympia Geghamyan, Davit Zakaryan, Zara Ohanjanyan International consultant: Soroosh Sorooshian 2 Content List of Abbreviations ............................................................................................................................... 7 Executive Summary ............................................................................................................................... 12 CHAPTER 1. ANALYSIS OF POLICY, LEGAL AND INSTITUTIONAL FRAMEWORK OF WATER SECTOR AND IDENTIFICATION OF GAPS AND BARRIERS IN THE CONTEXT OF CLIMATE CHANGE ............................. 19 Summary of Chapter 1 .......................................................................................................................... 19 1.1 The concept and criteria of water resources adaptation to climate change ................................. -

Armenian Tourist Attraction
Armenian Tourist Attractions: Rediscover Armenia Guide http://mapy.mk.cvut.cz/data/Armenie-Armenia/all/Rediscover%20Arme... rediscover armenia guide armenia > tourism > rediscover armenia guide about cilicia | feedback | chat | © REDISCOVERING ARMENIA An Archaeological/Touristic Gazetteer and Map Set for the Historical Monuments of Armenia Brady Kiesling July 1999 Yerevan This document is for the benefit of all persons interested in Armenia; no restriction is placed on duplication for personal or professional use. The author would appreciate acknowledgment of the source of any substantial quotations from this work. 1 von 71 13.01.2009 23:05 Armenian Tourist Attractions: Rediscover Armenia Guide http://mapy.mk.cvut.cz/data/Armenie-Armenia/all/Rediscover%20Arme... REDISCOVERING ARMENIA Author’s Preface Sources and Methods Armenian Terms Useful for Getting Lost With Note on Monasteries (Vank) Bibliography EXPLORING ARAGATSOTN MARZ South from Ashtarak (Maps A, D) The South Slopes of Aragats (Map A) Climbing Mt. Aragats (Map A) North and West Around Aragats (Maps A, B) West/South from Talin (Map B) North from Ashtarak (Map A) EXPLORING ARARAT MARZ West of Yerevan (Maps C, D) South from Yerevan (Map C) To Ancient Dvin (Map C) Khor Virap and Artaxiasata (Map C Vedi and Eastward (Map C, inset) East from Yeraskh (Map C inset) St. Karapet Monastery* (Map C inset) EXPLORING ARMAVIR MARZ Echmiatsin and Environs (Map D) The Northeast Corner (Map D) Metsamor and Environs (Map D) Sardarapat and Ancient Armavir (Map D) Southwestern Armavir (advance permission -
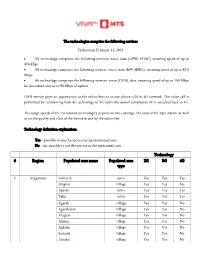
Technology # Region Populated Area Name Populated Area Type 2G 3G 4G
The technologies comprise the following services Updated on February 11, 2019 2G technology comprises the following services: voice, data (GPRS, EDGE), ensuring speed of up to 474 Kbps 3G technology comprises the following services: voice, data (R99, HSPA), ensuring speed of up to 42.2 Mbps 4G technology comprises the following services: voice (CSFB), data, ensuring speed of up to 150 Mbps for download and up to 50 Mbps of upload CSFB service gives an opportunity to the subscribers to accept phone calls in 4G network. The voice call is performed by transferring from 4G technology to 3G; upon the session completion 3G is switched back to 4G. The usage speeds of the mentioned technologies depend on the coverage, the load of the base station as well as on the quality and class of the device in use by the subscriber. Technology definition explanation: Yes – possible to use the service in the mentioned area No - not possible to use the service in the mentioned area Technology # Region Populated area name Populated area 2G 3G 4G type 1 Aragatsotn Ashtarak town Yes Yes Yes Mughni village Yes Yes No Aparan town Yes Yes Yes Talin town Yes Yes Yes Agarak village Yes Yes No Agarakavan village Yes Yes No Alagyaz village Yes Yes No Akunq village Yes Yes No Aghdzq village Yes Yes No Sadunts village Yes Yes No Antarut village Yes Yes No Ashnak village Yes Yes No Avan village Yes Yes No Khnusik village No No No Metsadzor village Yes No No Avshen village Yes Yes No Aragats village Yes Yes No Aragatsavan village Yes Yes No Aragatsotn village Yes Yes -

HAYK's SPIRIT IS IMMORTAL More Than 4500-Year-Old Roots of The
HAYK’S SPIRIT IS IMMORTAL Danielyan E. L. Doctor of Sciences (History) ETERNAL GLORY AND HONOR TO THE HEROES AND THEIR COMRADES-IN-ARMS WHO SACRIFICED THEIR LIVES FOR THE FREEDOM AND INDEPENDENCE OF THE FATHERLAND More than 4500-year-old roots of the Armenian Army are hallowed by the freedom struggle of the Armenian nation for the defence of the Fatherland against foreign invaders. The Armenian liberation torch sanctified by Hayk Nahapet (Patriarch) passed over from Hayots Dzor1 to Avarayr, Zeytun, Sasun, Sardarapat and has reached Artsakh. The heroes sacrificing their lives for the liberation of the Fatherland are immortalized. Hayk Lake Van 1 Մովսէս Խորենացի, Պատմութիւն Հայոց, Երևան, 1991, էջ 32-37: The year 2008 marked the 4500th anniversary of the victory of the Armenian Patriarch Hayk against Bel at the battle of Hayots Dzor (on the shore of Lake Van). Thе calendar calculation of the date based on the periodicity of “Hayk’s Cycle” of the “Ancient Armenian era” was done by the famous Armenologist Ghevond Alishan (1820-1901) (Ալիշան Ղ., Յուշիկք հայրենեաց Հայոց, հ. Ա, Վենետիկ, 1920, էջ 85). There was no leap-year in the ancient Armenian era, since a year was always considered to consist of 365 days; hence the year and the date were movable. Thus 1460 years, according to the Julian calendar, amount to 1461 years, according to the Armenian Calendar. By such periodicity of the “Cycle of Hayk”, 2492 BC denotes the year of Hayk’s victory. The beginning of the victorious year was Navasard 1 (=August 11). New discoveries connected with the observations of the starry sky (the 6th millennium BC - Zorats kar (Karahunj), the first half of the 3rd millennium BC - Metsamor), archaeological excavations and petroglyphs in the Armenian Highland, bear witness to the deep Haykian roots, and that the glorious victory of Hayk symbolized the beginning of a very important new epoch of the Armenian history. -

Exclusiveparliamentary Elections: Armenia at 25
EXCLUSIVE PARLIAMENTARY ELECTIONS: ARMENIA AT 25 FACES THE FUTURE P.20 ARMENIAN GENERAL BENEVOLENT UNION FEB. 2017 The Promise Overcoming the obstacles as epic story of survival and compassion starring Christian Bale and Oscar Isaac hits theaters April 21 Armenian General Benevolent Union ESTABLISHED IN 1906 Central Board of Directors Հայկական Բարեգործական Ընդհանուր Միութիւն President Mission Berge Setrakian To preserve and promote the Armenian heritage through worldwide educational, cul- Vice Presidents tural and humanitarian programs Sam Simonian Sinan Sinanian Annual International Budget Treasurer Forty-six million dollars (USD) Nazareth A. Festekjian Assistant Treasurer Education Yervant Demirjian 24 primary, secondary, preparatory and Saturday schools; scholarships; alternative edu- Secretary cational resources (apps, e-books, AGBU WebTalks & more); American University of Armenia; Armenian Virtual College (AVC); TUMO x AGBU Sarkis Jebejian Assistant Secretary Cultural, Humanitarian and Religious Arda Haratunian AGBU News Magazine; the AGBU Humanitarian Emergency Relief Fund for Syrian Honorary Member Armenians; athletics; camps; choral groups; concerts; dance; films; lectures; leadership; His Holiness Karekin II, library research centers; medical centers; mentorships; music competitions; publica- Armenia: Catholicos of all Armenians tions; radio; scouts; summer internships; theater; youth trips to Armenia. Members Holy Etchmiadzin; Arapkir, Malatya and Nork Children’s Centers and Senior Dining UNITED STATES Centers; Hye Geen Women’s -

UNFPA Armenia Country Programme Evaluation
Third UNFPA Country Programme: Armenia 2016-2020 FINAL EVALUATION REPORT November 2019 Nov Source: https://www.un.org/Depts/Cartographic/map/profile/armenia.pdf r 20November 2016 Country Programme Evaluation: Armenia The analysis and recommendations of this report do not necessarily reflect the views of the United Nations Population Fund, its Executive Board or the United Nations Member States. EVALUATION TEAM Team Leader Arlette Campbell White Evaluator Ada Babloyan Evaluator Lusine Kharatyan Evaluation Research Assistant and Interpreter Manana Mananyan 2 UNFPA Armenia CO Country Programme Evaluations Reference Group Composition Name Organisation 1. Mahbub Alam M&E Adviser, UNFPA 2. Tsovinar Harutyunyan Assistant Representative, UNFPA Armenia CO 3. Lusine Sargsyan Evaluation Manager, UNFPA Armenia CO 4. Anahit Safyan National Statistical Committee 5. Anahit Martirosyan Ministry of Labour and Social Issues 6. Zhanna Andreasyan Ministry of Labour and Social Issues 7. Nune Pashayan Head of the Mother and childcare department, Ministry of Health 8. Arman Hovhannisyan Head of UN Desk MFA Armenia 9. Mane Tadevosyan RC Office, Monitoring and Evaluation 10. Mikayel Khachatryan Human Rights Defender’s Office 11. Nelly Duryan RA Police 12. Anna Harutyunyan Individual consultant 13. Astghik Martirosyan Monitoring and Evaluation/Child Rights Systems Monitoring Specialist , UNICEF Acknowledgements The Evaluation Team would like to thank UNFPA for the opportunity to undertake the evaluation for the Government of the Republic of Armenia and UNFPA’s Third Country Programme. We are particularly grateful to the UNFPA Armenia Country Office staff members who, despite a very heavy workload and other commitments, were so generous with their time and responsive to the Team’s repeated requests, often at short notice. -

UNDP Armenia 'Women in Local Development: Women in Politics'
UNDP Armenia ‘Women in Local Development: Women in Politics’ project (https://open.undp.org/projects/00110249 ) Mid-Term Evaluation Report 23.02.2021 Report produced by Roderick Ackermann for UNDP Armenia Project and evaluation information Project/outcome Information Project/outcome title ‘Women in Politics’ project/ Project Document title: ‘Women in Local Development’ Atlas ID 00110249- 00109276 Corporate outcome and • 2016-20 UNDAF Outcome 3: output • 2016-2020 UNDP Country Programme Action Plan Outcome 3 (12) • 2018-2021 UNDP Strategic Plan Output 1.6.1 Country Armenia Region Europe and CIS Date project document Undated signed Project dates Start Planned end November 2018 March 2021, extended to September 2021 Project budget Initial budget: USD 1,451,373 (GBP 1,108,849) Revised budget: USD… (GBP 1,253,620) Project expenditure at the USD 977,154 time of evaluation Funding source UK Department for International Development Implementing party UNDP Armenia & OxYGen Foundation Evaluation type (project/ Project outcome/thematic/country programme, etc.) Final/midterm review/ other Midterm Period under evaluation Start End November 2018 November 2020 Evaluators Roderick Ackermann Evaluator email address [email protected] Evaluation dates Start Completion 06/10/2020 11/12/2020 Contents Project and evaluation information ...................................................................................................................... 2 Contents ............................................................................................................................................................... -

WORLD COUNCIL of CHURCHES Armenia Round Table
WORLD COUNCIL OF CHURCHES Armenia Round Table ANNUAL REPORT 2006 EDUCATIONAL PROGRAMME AGRICULTURAL DEVELOPMENT PROGRAMME CAPACITY BUILDING PROGRAMME 2006 WCC Armenia Round Table World Council of Churches Monastery of Etchmiadzin WCC Diakonia & Solidarity Team - Europe Desk Tel: +374 (10) 517 157 150, Route de Ferney, P.O. Box 2100, CH -1211 Geneva 2 Fax: +374 (10) 517 436 Tel: +41 22 791 6210 and 791 6209 E-mail: [email protected] Fax: +41 22 788 0067 Web page: www.armwcc.org E-mail: [email protected], [email protected] ANNUAL REPORT Web page: www.wcc-coe.org/wcc/europe WORLD COUNCIL OF CHURCHES ARMENIA ROUND TABLE CONTENTS Introductory remarks ...................................................................5 EDUCATIONAL PROGRAMME ...................................................10 Christian Education and Social Diakonia ...............................12 Training Courses ....................................................................18 Teachers’ Training Programme and Social Service Centres ............................................................23 Participation in National Educational Policy Discussions and Policy Advocacy for Involvement of Churches in Education and Social Services .................................................27 AGRICULTURAL DEVELOPMENT PROGRAMME .......................30 Promotion of Demonstration/ Monastery Farms ...................................................................32 Promotion of Sustainable Agricultural Development ..............36 Support of Locally Rooted Solutions with a Poverty