NEA Shark Covers
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Centroscymnus Coelolepis)
W&M ScholarWorks VIMS Articles Virginia Institute of Marine Science 2011 Population structure of a deep-water squaloid shark, the Portuguese dogfish (Centroscymnus coelolepis) A Verissimo Virginia Institute of Marine Science Jan McDowell Virginia Institute of Marine Science John Graves Virginia Institute of Marine Science Follow this and additional works at: https://scholarworks.wm.edu/vimsarticles Part of the Aquaculture and Fisheries Commons Recommended Citation Verissimo, A; McDowell, Jan; and Graves, John, "Population structure of a deep-water squaloid shark, the Portuguese dogfish (Centroscymnus coelolepis)" (2011). VIMS Articles. 932. https://scholarworks.wm.edu/vimsarticles/932 This Article is brought to you for free and open access by the Virginia Institute of Marine Science at W&M ScholarWorks. It has been accepted for inclusion in VIMS Articles by an authorized administrator of W&M ScholarWorks. For more information, please contact [email protected]. ICES Journal of Marine Science (2011), 68(3), 555–563. doi:10.1093/icesjms/fsr003 Population structure of a deep-water squaloid shark, the Portuguese dogfish (Centroscymnus coelolepis) Downloaded from https://academic.oup.com/icesjms/article-abstract/68/3/555/661444 by Serials Dept -- College of William and Mary user on 02 November 2018 Ana Verı´ssimo*, Jan R. McDowell, and John E. Graves Virginia Institute of Marine Science, College of William & Mary, PO Box 1346, Gloucester Point, VA 23062, USA *Corresponding Author: tel: +1 804 684 7434; fax: +1 804 684 7157; e-mail: [email protected]. Verı´ssimo, A., McDowell, J. R., and Graves, J. E. 2011. Population structure of a deep-water squaloid shark, the Portuguese dogfish (Centroscymnus coelolepis). -

Extinction Risk and Conservation of the World's Sharks and Rays
RESEARCH ARTICLE elife.elifesciences.org Extinction risk and conservation of the world’s sharks and rays Nicholas K Dulvy1,2*, Sarah L Fowler3, John A Musick4, Rachel D Cavanagh5, Peter M Kyne6, Lucy R Harrison1,2, John K Carlson7, Lindsay NK Davidson1,2, Sonja V Fordham8, Malcolm P Francis9, Caroline M Pollock10, Colin A Simpfendorfer11,12, George H Burgess13, Kent E Carpenter14,15, Leonard JV Compagno16, David A Ebert17, Claudine Gibson3, Michelle R Heupel18, Suzanne R Livingstone19, Jonnell C Sanciangco14,15, John D Stevens20, Sarah Valenti3, William T White20 1IUCN Species Survival Commission Shark Specialist Group, Department of Biological Sciences, Simon Fraser University, Burnaby, Canada; 2Earth to Ocean Research Group, Department of Biological Sciences, Simon Fraser University, Burnaby, Canada; 3IUCN Species Survival Commission Shark Specialist Group, NatureBureau International, Newbury, United Kingdom; 4Virginia Institute of Marine Science, College of William and Mary, Gloucester Point, United States; 5British Antarctic Survey, Natural Environment Research Council, Cambridge, United Kingdom; 6Research Institute for the Environment and Livelihoods, Charles Darwin University, Darwin, Australia; 7Southeast Fisheries Science Center, NOAA/National Marine Fisheries Service, Panama City, United States; 8Shark Advocates International, The Ocean Foundation, Washington, DC, United States; 9National Institute of Water and Atmospheric Research, Wellington, New Zealand; 10Global Species Programme, International Union for the Conservation -

© Iccat, 2007
A5 By-catch Species APPENDIX 5: BY-CATCH SPECIES A.5 By-catch species By-catch is the unintentional/incidental capture of non-target species during fishing operations. Different types of fisheries have different types and levels of by-catch, depending on the gear used, the time, area and depth fished, etc. Article IV of the Convention states: "the Commission shall be responsible for the study of the population of tuna and tuna-like fishes (the Scombriformes with the exception of Trichiuridae and Gempylidae and the genus Scomber) and such other species of fishes exploited in tuna fishing in the Convention area as are not under investigation by another international fishery organization". The following is a list of by-catch species recorded as being ever caught by any major tuna fishery in the Atlantic/Mediterranean. Note that the lists are qualitative and are not indicative of quantity or mortality. Thus, the presence of a species in the lists does not imply that it is caught in significant quantities, or that individuals that are caught necessarily die. Skates and rays Scientific names Common name Code LL GILL PS BB HARP TRAP OTHER Dasyatis centroura Roughtail stingray RDC X Dasyatis violacea Pelagic stingray PLS X X X X Manta birostris Manta ray RMB X X X Mobula hypostoma RMH X Mobula lucasana X Mobula mobular Devil ray RMM X X X X X Myliobatis aquila Common eagle ray MYL X X Pteuromylaeus bovinus Bull ray MPO X X Raja fullonica Shagreen ray RJF X Raja straeleni Spotted skate RFL X Rhinoptera spp Cownose ray X Torpedo nobiliana Torpedo -

(Squalus Acanthias) and Black Dogfish (Centroscyllium Fabricii) Spanish Data (Surveys and Fishery) in NAFO Divisions 3LMNO
NOT TO BE CITED WITHOUT PRIOR REFERENCE TO THE SECRETARIAT Northwest Atlantic Fisheries Organization Serial No. N5250 NAFO SCR Doc. 06/30 SCIENTIFIC COUNCIL MEETING – JUNE 2006 Spiny Dogfish (Squalus acanthias) and Black Dogfish (Centroscyllium fabricii) Spanish Data (Surveys and Fishery) in NAFO Divisions 3LMNO. by F. González-Costas1, D. González-Troncoso1, M. Casas1 and G. Ramilo1 1 Instituto Español de Oceanografía, Vigo, Spain ABSTRACT The analysis of Spanish survey and fishery data from Divisions 3LMNO show that Spiny dogfish (Squalus acanthias) is not abundant and that this species appears in these Divisions sporadically and in depths of less than 500 meters. Black dogfish (Centroscyllium fabricii) data show that this species is present in all Divisions, but is more abundant in Div. 3NO and in depths of more than 900 m. Biomass estimated from the 3NO survey displays an increasing trend over the last three years. Commercial catches of this species are mainly a by-catch of the Greenland halibut fishery in Div. 3LMNO. Size compositions are mainly in between 50 and 70 cm of length, both for commercial and survey catch es. INTRODUCTION The aim of this paper is to review and present the Spanish information from surveys and commercial data for Spiny dogfish (Squalus acanthias) and Black dogfish (Centroscyllium fabricii) that were requested to the NAFO Scientific Council, in accord with the recommendation from the 2002 NAFO Symposium on Elasmobranches Fisheries. Part of this information had been presented by P. Duran et al. in 1999 for the period 1999-1998. MATERIAL AND METHODS Two sources of information have been used in this paper, data recorded by the National Scientific Observers and research survey dat a. -

APPENDIX M Common and Scientific Species Names
Bay du Nord Development Project Environmental Impact Statement APPENDIX M Common and Scientific Species Names Bay du Nord Development Project Environmental Impact Statement Common and Species Names Common Name Scientific Name Fish Abyssal Skate Bathyraja abyssicola Acadian Redfish Sebastes fasciatus Albacore Tuna Thunnus alalunga Alewife (or Gaspereau) Alosa pseudoharengus Alfonsino Beryx decadactylus American Eel Anguilla rostrata American Plaice Hippoglossoides platessoides American Shad Alosa sapidissima Anchovy Engraulidae (F) Arctic Char (or Charr) Salvelinus alpinus Arctic Cod Boreogadus saida Atlantic Bluefin Tuna Thunnus thynnus Atlantic Cod Gadus morhua Atlantic Halibut Hippoglossus hippoglossus Atlantic Mackerel Scomber scombrus Atlantic Salmon (landlocked: Ouananiche) Salmo salar Atlantic Saury Scomberesox saurus Atlantic Silverside Menidia menidia Atlantic Sturgeon Acipenser oxyrhynchus oxyrhynchus Atlantic Wreckfish Polyprion americanus Barndoor Skate Dipturus laevis Basking Shark Cetorhinus maximus Bigeye Tuna Thunnus obesus Black Dogfish Centroscyllium fabricii Blue Hake Antimora rostrata Blue Marlin Makaira nigricans Blue Runner Caranx crysos Blue Shark Prionace glauca Blueback Herring Alosa aestivalis Boa Dragonfish Stomias boa ferox Brook Trout Salvelinus fontinalis Brown Bullhead Catfish Ameiurus nebulosus Burbot Lota lota Capelin Mallotus villosus Cardinal Fish Apogonidae (F) Chain Pickerel Esox niger Common Grenadier Nezumia bairdii Common Lumpfish Cyclopterus lumpus Common Thresher Shark Alopias vulpinus Crucian Carp -
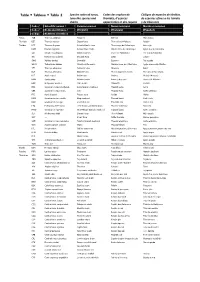
Table Tableau Tabla 2
Table Tableau Tabla 2 Species codes of tunas, Codes des espèces de Códigos de especies de túnidos, tuna‐like species and thonidés, d’espèces de especies afines a los túnidos sharks apparentées et des requins y de tiburones Code / Scientific names / Common names Noms communs Nombres comunes Code / Noms sientifiques / (English) (Français) (Español) Código Nombres científicos Tunas ALB Thunnus alalunga Albacore Germon Atún blanco Thonidés BET Thunnus obesus Bigeye tuna Thon obèse(=Patudo) Patudo Túnidos BFT Thunnus thynnus Atlantic bluefin tuna Thon rouge de l’atlantique Atún rojo BUM Makaira nigricans Atlantic blue marlin Makaire bleu de l'Atlantique Aguja azul del Atlántico SAI Istiophorus albicans Atlantic sailfish Voilier de l'Atlantique Pez vela del Atlántico SKJ Katsuwonus pelamis Skipjack tuna Listao Listado SWO Xiphias gladius Swordfish Espadon Pez espada WHM Tetrapturus albidus Atlantic white marlin Makaire blanc de l'Atlantique Aguja blanca del Atlántico YFT Thunnus albacares Yellowfin tuna Albacore Rabil BLF Thunnus atlanticus Blackfin tuna Thon à nageoires noires Atún des aletas negras BLT Auxis rochei Bullet tuna Bonitou Melva(=Melvera) BON Sarda sarda Atlantic bonito Bonite à dos rayé Bonito del Atlántico BOP Orcynopsis unicolor Plain bonito Palomette Tasarte BRS Scomberomorus brasiliensis Serra Spanish mackerel Thazard serra Serra CER Scomberomorus regalis Cero Thazard franc Carite chinigua FRI Auxis thazard Frigate tuna Auxide Melva KGM Scomberomorus cavalla King mackerel Thazard barré Carite lucio KGX Scomberomorus spp -

Identification Guide to the Deep-Sea Cartilaginous Fishes Of
Identification guide to the deep–sea cartilaginous fishes of the Southeastern Atlantic Ocean FAO. 2015. Identification guide to the deep–sea cartilaginous fishes of the Southeastern Atlantic Ocean. FishFinder Programme, by Ebert, D.A. and Mostarda, E., Rome, Italy. Supervision: Merete Tandstad, Jessica Sanders (FAO, Rome) Technical editor: Edoardo Mostarda (FAO, Rome) Colour illustrations, cover and graphic design: Emanuela D’Antoni (FAO, Rome) This guide was prepared under the “FAO Deep–sea Fisheries Programme” thanks to a generous funding from the Government of Norway (Support to the implementation of the International Guidelines on the Management of Deep-Sea Fisheries in the High Seas project) for the purpose of assisting states, institutions, the fishing industry and RFMO/As in the implementation of FAO International Guidelines for the Management of Deep-sea Fisheries in the High Seas. It was developed in close collaboration with the FishFinder Programme of the Marine and Inland Fisheries Branch, Fisheries Department, Food and Agriculture Organization of the United Nations (FAO). The present guide covers the deep–sea Southeastern Atlantic Ocean and that portion of Southwestern Indian Ocean from 18°42’E to 30°00’E (FAO Fishing Area 47). It includes a selection of cartilaginous fish species of major, moderate and minor importance to fisheries as well as those of doubtful or potential use to fisheries. It also covers those little known species that may be of research, educational, and ecological importance. In this region, the deep–sea chondrichthyan fauna is currently represented by 50 shark, 20 batoid and 8 chimaera species. This guide includes full species accounts for 37 shark, 9 batoid and 4 chimaera species selected as being the more difficult to identify and/or commonly caught. -

Coelho Phd Lantern S
UNIVERSIDADEdo ALGARVE FaculdadedeCiênciasdoMaredo Ambiente Biology,populationdynamics,managementandconservation ofdeepwaterlanternsharks,Etmopterusspinax and Etmopteruspusillus (Chondrichthyes:Etmopteridae)insouthernPortugal(northeastAtlantic). (DoutoramentoemCiênciaseTecnologiasdasPescas,especialidadedeBiologiaPesqueira) (ThesisforthedegreeinDoctorofPhilosophyinFisheriesSciencesandTechnologies,specialtyinFisheriesBiology) RUIPEDROANDRADECOELHO Faro (2007) UNIVERSIDADE DO ALGARVE FACULDADE DE CIÊNCIAS DO MAR E DO AMBIENTE Biology, population dynamics, management and conservation of deep water lantern sharks, Etmopterus spinax and Etmopterus pusillus (Chondrichthyes: Etmopteridae) in southern Portugal (northeast Atlantic). (Doutoramento em Ciências e Tecnologias das Pescas, especialidade de Biologia Pesqueira) (Thesis for the degree in Doctor of Philosophy in Fisheries Sciences and Technologies, specialty in Fisheries Biology) RUI PEDRO ANDRADE COELHO Orientador / Supervisor: Prof. Doutor Karim Erzini Júri / Jury: - Prof. Doutor José Pedro Andrade, Professor Catedrático da Faculdade de Ciências do Mar e do Ambiente, Universidade do Algarve; - Prof. Doutor Karim Erzini, Professor Associado com Agregação da Faculdade de Ciências do Mar e do Ambiente, Universidade do Algarve; - Prof. Doutor Leonel Paulo Sul de Serrano Gordo, Professor Auxiliar com Agregação da Faculdade de Ciências, Universidade de Lisboa; - Prof. Doutor Manuel Seixas Afonso Dias, Professor Auxiliar da Faculdade de Ciências do Mar e do Ambiente, Universidade do Algarve; -

Report on the Status of Mediterranean Chondrichthyan Species
United Nations Environment Programme Mediterranean Action Plan Regional Activity Centre For Specially Protected Areas REPORT ON THE STATUS OF MEDITERRANEAN CHONDRICHTHYAN SPECIES D. CEBRIAN © L. MASTRAGOSTINO © R. DUPUY DE LA GRANDRIVE © Note : The designations employed and the presentation of the material in this document do not imply the expression of any opinion whatsoever on the part of UNEP concerning the legal status of any State, Territory, city or area, or of its authorities, or concerning the delimitation of their frontiers or boundaries. © 2007 United Nations Environment Programme Mediterranean Action Plan Regional Activity Centre for Specially Protected Areas (RAC/SPA) Boulevard du leader Yasser Arafat B.P.337 –1080 Tunis CEDEX E-mail : [email protected] Citation: UNEP-MAP RAC/SPA, 2007. Report on the status of Mediterranean chondrichthyan species. By Melendez, M.J. & D. Macias, IEO. Ed. RAC/SPA, Tunis. 241pp The original version (English) of this document has been prepared for the Regional Activity Centre for Specially Protected Areas (RAC/SPA) by : Mª José Melendez (Degree in Marine Sciences) & A. David Macías (PhD. in Biological Sciences). IEO. (Instituto Español de Oceanografía). Sede Central Spanish Ministry of Education and Science Avda. de Brasil, 31 Madrid Spain [email protected] 2 INDEX 1. INTRODUCTION 3 2. CONSERVATION AND PROTECTION 3 3. HUMAN IMPACTS ON SHARKS 8 3.1 Over-fishing 8 3.2 Shark Finning 8 3.3 By-catch 8 3.4 Pollution 8 3.5 Habitat Loss and Degradation 9 4. CONSERVATION PRIORITIES FOR MEDITERRANEAN SHARKS 9 REFERENCES 10 ANNEX I. LIST OF CHONDRICHTHYAN OF THE MEDITERRANEAN SEA 11 1 1. -

FISHES (C) Val Kells–November, 2019
VAL KELLS Marine Science Illustration 4257 Ballards Mill Road - Free Union - VA - 22940 www.valkellsillustration.com [email protected] STOCK ILLUSTRATION LIST FRESHWATER and SALTWATER FISHES (c) Val Kells–November, 2019 Eastern Atlantic and Gulf of Mexico: brackish and saltwater fishes Subject to change. New illustrations added weekly. Atlantic hagfish, Myxine glutinosa Sea lamprey, Petromyzon marinus Deepwater chimaera, Hydrolagus affinis Atlantic spearnose chimaera, Rhinochimaera atlantica Nurse shark, Ginglymostoma cirratum Whale shark, Rhincodon typus Sand tiger, Carcharias taurus Ragged-tooth shark, Odontaspis ferox Crocodile Shark, Pseudocarcharias kamoharai Thresher shark, Alopias vulpinus Bigeye thresher, Alopias superciliosus Basking shark, Cetorhinus maximus White shark, Carcharodon carcharias Shortfin mako, Isurus oxyrinchus Longfin mako, Isurus paucus Porbeagle, Lamna nasus Freckled Shark, Scyliorhinus haeckelii Marbled catshark, Galeus arae Chain dogfish, Scyliorhinus retifer Smooth dogfish, Mustelus canis Smalleye Smoothhound, Mustelus higmani Dwarf Smoothhound, Mustelus minicanis Florida smoothhound, Mustelus norrisi Gulf Smoothhound, Mustelus sinusmexicanus Blacknose shark, Carcharhinus acronotus Bignose shark, Carcharhinus altimus Narrowtooth Shark, Carcharhinus brachyurus Spinner shark, Carcharhinus brevipinna Silky shark, Carcharhinus faiformis Finetooth shark, Carcharhinus isodon Galapagos Shark, Carcharhinus galapagensis Bull shark, Carcharinus leucus Blacktip shark, Carcharhinus limbatus Oceanic whitetip shark, -

Proteoglycans Isolated from Bramble Shark
mac har olo P gy : & O y r p t e s i n A m c e Ajeeshkumar et al., Biochem Pharmacol 2017, 6:2 c h e c s Open Access o i s Biochemistry & Pharmacology: B DOI: 10.4172/2167-0501.1000226 ISSN: 2167-0501 Research Article Open Access Proteoglycans Isolated from Bramble Shark Cartilage (Echinorhinus brucus) Inhibits Proliferation of MCF-7 Human Breast Cancer Cells by Inducing Apoptosis Ajeeshkumar KK1*, Asha KK1, Vishnu KV1, Remyakumari KR1, Shyni K1, Reshma J2, Navaneethan R1, Linu B3 and Mathew S1 1Biochemistry and Nutrition Division, Central Institute of Fisheries Technology, Cochin-29, Kerala, India 2Department of Biochemistry, SNGIST Arts and Science College (SNGIST ASc.), Affiliated to Mahatma Gandhi University, Kerala, India 3National Centre for Aquatic Animal Health, Cochin University of Science and Technology, Cochin, Kerala, India *Corresponding author: Ajeeshkumar KK, Biochemistry and Nutrition Division, Central Institute of Fisheries Technology, Cochin-29, Kerala, India, Tel: +91-9747304434; E-mail: [email protected] Receiving date: June 22, 2017; Acceptance date: July 03, 2017; Publication date: July 24, 2017 Copyright: © 2017 Ajeeshkumar KK, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Abstract Proteoglycans (PGs) were isolated from deep sea shark Echinorhinus brucus (bramble shark) cartilage and their anti-proliferative activity against MCF-7 cell lines was evaluated. To establish apoptosis involvement for cytotoxicity, the following assays were performed i.e., caspases 3 and 9 assay, double fluorescent staining and DNA laddering assay. -

Tropical Eastern Pacific Records of the Prickly Shark, Echinorhinus Cookei
Tropical Eastern Pacific Records of the Prickly Shark, Echinorhinus cookei (Chondrichthyes: Echinorhinidae)1 Douglas J. Long,2,3,5 John E. McCosker,3 Shmulik Blum,4 and Avi Klapfer4 Abstract: Most records of the prickly shark, Echinorhinus cookei Pietschmann, 1928, are from temperate and subtropical areas of the Pacific rim, with few rec- ords from the tropics. This seemingly disjunct distribution led some authors to consider E. cookei to have an antitropical distribution. Unreported museum spec- imens and underwater observations of E. cookei from Cocos Island, Costa Rica; the Galápagos Islands; and northern Peru confirm its occurrence in the trop ical eastern Pacific and, combined with other published records from the eastern Pacific, establish a continuous, panhemispheric eastern Pacific distribution. The genus Echinorhinus contains two spe- Mundy [1994] and Crow et al. [1996]); it has cies, the bramble shark, E. brucus (Bonnaterre, subsequently been collected or observed off 1788), from the Atlantic, Mediterranean, Japan (Taniuchi and Yanagisawa 1983, Ko- western Indian Ocean, and Australia, New bayashi 1986), Taiwan (Teng 1958), Palau Zealand, and Japan, and the prickly shark, (Saunders 1984), Tonga (Randall et al. 2003), E. cookei Pietschmann, 1928, known from New Caledonia (Fourmanoir 1979), New Hawai‘i and the western and eastern Pacific Zealand (Garrick 1960, Garrick and More- Ocean (Compagno et al. 2005, Last and Ste- land 1968), northeastern and southeastern vens 2009). The species are easily differenti- Australia (Last and Stevens 2009), and possi- ated by visual examination: E. brucus possesses bly the Gilbert Islands ( Whitley and Colefax few, relatively large, sparse denticles, some of 1938). In the northeastern Pacific it was first which are fused into plates, and E.