Centroscymnus Coelolepis)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

APPENDIX M Common and Scientific Species Names
Bay du Nord Development Project Environmental Impact Statement APPENDIX M Common and Scientific Species Names Bay du Nord Development Project Environmental Impact Statement Common and Species Names Common Name Scientific Name Fish Abyssal Skate Bathyraja abyssicola Acadian Redfish Sebastes fasciatus Albacore Tuna Thunnus alalunga Alewife (or Gaspereau) Alosa pseudoharengus Alfonsino Beryx decadactylus American Eel Anguilla rostrata American Plaice Hippoglossoides platessoides American Shad Alosa sapidissima Anchovy Engraulidae (F) Arctic Char (or Charr) Salvelinus alpinus Arctic Cod Boreogadus saida Atlantic Bluefin Tuna Thunnus thynnus Atlantic Cod Gadus morhua Atlantic Halibut Hippoglossus hippoglossus Atlantic Mackerel Scomber scombrus Atlantic Salmon (landlocked: Ouananiche) Salmo salar Atlantic Saury Scomberesox saurus Atlantic Silverside Menidia menidia Atlantic Sturgeon Acipenser oxyrhynchus oxyrhynchus Atlantic Wreckfish Polyprion americanus Barndoor Skate Dipturus laevis Basking Shark Cetorhinus maximus Bigeye Tuna Thunnus obesus Black Dogfish Centroscyllium fabricii Blue Hake Antimora rostrata Blue Marlin Makaira nigricans Blue Runner Caranx crysos Blue Shark Prionace glauca Blueback Herring Alosa aestivalis Boa Dragonfish Stomias boa ferox Brook Trout Salvelinus fontinalis Brown Bullhead Catfish Ameiurus nebulosus Burbot Lota lota Capelin Mallotus villosus Cardinal Fish Apogonidae (F) Chain Pickerel Esox niger Common Grenadier Nezumia bairdii Common Lumpfish Cyclopterus lumpus Common Thresher Shark Alopias vulpinus Crucian Carp -

Identification Guide to the Deep-Sea Cartilaginous Fishes Of
Identification guide to the deep–sea cartilaginous fishes of the Southeastern Atlantic Ocean FAO. 2015. Identification guide to the deep–sea cartilaginous fishes of the Southeastern Atlantic Ocean. FishFinder Programme, by Ebert, D.A. and Mostarda, E., Rome, Italy. Supervision: Merete Tandstad, Jessica Sanders (FAO, Rome) Technical editor: Edoardo Mostarda (FAO, Rome) Colour illustrations, cover and graphic design: Emanuela D’Antoni (FAO, Rome) This guide was prepared under the “FAO Deep–sea Fisheries Programme” thanks to a generous funding from the Government of Norway (Support to the implementation of the International Guidelines on the Management of Deep-Sea Fisheries in the High Seas project) for the purpose of assisting states, institutions, the fishing industry and RFMO/As in the implementation of FAO International Guidelines for the Management of Deep-sea Fisheries in the High Seas. It was developed in close collaboration with the FishFinder Programme of the Marine and Inland Fisheries Branch, Fisheries Department, Food and Agriculture Organization of the United Nations (FAO). The present guide covers the deep–sea Southeastern Atlantic Ocean and that portion of Southwestern Indian Ocean from 18°42’E to 30°00’E (FAO Fishing Area 47). It includes a selection of cartilaginous fish species of major, moderate and minor importance to fisheries as well as those of doubtful or potential use to fisheries. It also covers those little known species that may be of research, educational, and ecological importance. In this region, the deep–sea chondrichthyan fauna is currently represented by 50 shark, 20 batoid and 8 chimaera species. This guide includes full species accounts for 37 shark, 9 batoid and 4 chimaera species selected as being the more difficult to identify and/or commonly caught. -

Coelho Phd Lantern S
UNIVERSIDADEdo ALGARVE FaculdadedeCiênciasdoMaredo Ambiente Biology,populationdynamics,managementandconservation ofdeepwaterlanternsharks,Etmopterusspinax and Etmopteruspusillus (Chondrichthyes:Etmopteridae)insouthernPortugal(northeastAtlantic). (DoutoramentoemCiênciaseTecnologiasdasPescas,especialidadedeBiologiaPesqueira) (ThesisforthedegreeinDoctorofPhilosophyinFisheriesSciencesandTechnologies,specialtyinFisheriesBiology) RUIPEDROANDRADECOELHO Faro (2007) UNIVERSIDADE DO ALGARVE FACULDADE DE CIÊNCIAS DO MAR E DO AMBIENTE Biology, population dynamics, management and conservation of deep water lantern sharks, Etmopterus spinax and Etmopterus pusillus (Chondrichthyes: Etmopteridae) in southern Portugal (northeast Atlantic). (Doutoramento em Ciências e Tecnologias das Pescas, especialidade de Biologia Pesqueira) (Thesis for the degree in Doctor of Philosophy in Fisheries Sciences and Technologies, specialty in Fisheries Biology) RUI PEDRO ANDRADE COELHO Orientador / Supervisor: Prof. Doutor Karim Erzini Júri / Jury: - Prof. Doutor José Pedro Andrade, Professor Catedrático da Faculdade de Ciências do Mar e do Ambiente, Universidade do Algarve; - Prof. Doutor Karim Erzini, Professor Associado com Agregação da Faculdade de Ciências do Mar e do Ambiente, Universidade do Algarve; - Prof. Doutor Leonel Paulo Sul de Serrano Gordo, Professor Auxiliar com Agregação da Faculdade de Ciências, Universidade de Lisboa; - Prof. Doutor Manuel Seixas Afonso Dias, Professor Auxiliar da Faculdade de Ciências do Mar e do Ambiente, Universidade do Algarve; -

NEA Shark Covers
This report describes the results of a regional Red List Workshop held at the Joint Nature Conservation Committee (JNCC), Peterborough, UK, in 2006, as a contribution towards the IUCN Species Survival Commission’s Shark Specialist Group’s ‘Global Shark Red List Assessment’. The purpose of the workshop was to assess the conservation status of the chondrichthyan fishes (sharks, rays and chimaeras) of the Northeast Atlantic region (FAO Major Fishery Area 27). This region is bordered by some of the largest and most important chondrichthyan fishing nations in the world, including Spain, France, the UK and Portugal. A regional overview of fisheries, utilisation, trade, management and conservation is also presented. The Northeast Atlantic chondrichthyan fauna is moderately diverse, with an estimated 118 species (approximately 11% of total living chondrichthyans). These occur within a huge range of habitats, including the deep-sea, open oceans, and coastal waters from the Arctic to the Mediterranean. During the workshop, experts collated information and prepared 74 global and 17 regional species assessments, thereby completing the Red Listing process for the described chondrichthyan fauna of the Northeast Atlantic (two undescribed species were not assessed). These assessments were agreed by consensus throughout the SSG network prior to their submission to IUCN Red List of Threatened SpeciesTM. Results show that 26% of Northeast Atlantic chondrichthyans are threatened within the region (7% Critically Endangered, 7% Endangered, 12% Vulnerable). A further 20% are Near Threatened, 27% Least Concern and 27% are Data Deficient. This is a significantly higher level of threat than that for the whole taxonomic group, worldwide. Globally, of the 1, 038 species of chondrichthyans assessed, 18% are threatened (3% CR, 4% EN, 11% VU), 13% are Near Threatened, 23% Least Concern and 46% Data Deficient. -

5 November 1997
An identification guide for deepwater shark species Di Tracey and Peter Shearer National Institute of Water and Atmospheric Research August 2002 Contents Page Baxter’s dogfish (Etmopterus baxteri) 2 Catsharks (Apristurus spp.) 3 Leafscale gulper shark (Centrophorus squamosus) 4 Longnose velvet dogfish (Centroscymnus crepidater) 5 Lucifer dogfish (Etmopterus lucifer) 6 Northern spiny dogfish (Squalus mitsukurii) 7 Owston’s dogfish (Centroscymnus owstoni) 8 Plunket’s shark (Centroscymnus plunketi) 9 Portuguese dogfish (Centroscymnus coelolepis) 10 Prickly dogfish (Oxynotus bruniensis) 11 Seal shark (Dalatias licha) 12 Shovelnose dogfish (Deania calcea) 13 Spiny dogfish (Squalus acanthias) 14 NIWA Guide to Some Common New Zealand Deepwater Sharks – August 2002 Introduction The aim of this guide is to provide clear and concise identification sheets for deepwater shark species for future use by scientific observers and the fishing industry. This guide is an updated version of the Deepwater Shark Reference Guide sheets already prepared by NIWA. The shark species are presented alphabetically by common name. Layout for each species is consistent throughout the document and includes the following: • common name, scientific name, and species code • colour photograph and line drawing for each species • information on the depth distribution of the species • diagnostic features – outlines the key characteristics used to identify the species • maximum lengths that are attained • colour photograph and line drawing of the underside of head for some species These sheets can be substituted for the sheets already included in the revised Observer Manual. Enclosed is a CD ROM containing the shark guide as an interactive PDF document, an Acrobat Reader installer, and a word document. -
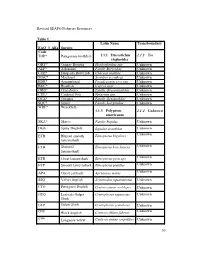
Revised SEAFO Fisheries Resources Table 1. . FAO 3 Alfa Species Code
Revised SEAFO Fisheries Resources Table 1. Latin Name Transboundary FAO 3 Alfa Species Code TOP* Patagonian toothfish 1.1.1 Dissostichus 1.1.2 Yes eleginoides ORY* Orange Roughy Hoplosthethus spp Unknown ALF* Alfonsino Family Berycidae Unknown CGE* Deep-sea Red Crab Chaceon maritae Unknown MAC* Mackerel Scomber scombrus Unknown EDR* Armourhead Pseudopentaceros spp. Unknown BOC* Boarfish Capros aper Unknown ORD* Oreo dories Family Oreosomatidae Unknown CDL* Cardinal Fish Epigonus spp. Unknown OCZ* Octopus Family Octopodidae Unknown SQC* Squid Family Loliginidae Unknown WRF* Wreckfish 1.1.3 Polyprion 1.1.4 Unknown americanus SKA* Skates Family Rajidae Unknown DGS Spiny Dogfish Squalus acanthias Unknown Unknown ETB Blurred smooth Etmopterus bigelowi lanternshark ETH Shorttail Etmopterus brachyurus Unknown lanternshark ETR Great lanternshark Etmopterus princeps Unknown ETP Smooth lanternshark Etmopterus pusillus Unknown Unknown APA Ghost catshark Apristurus manis SSQ Velvet dogfish Scymnodon squamulosus Unknown CYO Portuguese Dogfish Centroscymnus coelolepis Unknown GUQ Leafscale Gulper Centrophorus squamosus Unknown Shark GUP Gulper Shark Centrophorus granulosus Unknown CFB ǂ Black dogfish Centroscyllium fabricii Unknown CYP ǂ Longnose velvet Centroscymnus crepidater Unknown 30 dogfish CYY Unknown ǂ Shortnose velvet Centroscymnus dogfish cryptacanthus SCK ǂ Kitefin shark Dalatias licha Unknown ETE ǂ Etmopterus compagnoi Unknown ETI ǂ Broadbanded Etmopterus gracilispinis Unknown lanternshark ETM ǂ Unknown Southern Etmopterus granulosus lanternshark -

WKSHARK6 Report 2020
WORKSHOP ON THE DISTRIBUTION AND BYCATCH MANAGEMENT OPTIONS OF LISTED DEEP-SEA SHARK SPECIES (WKSHARK6) VOLUME 2 | ISSUE 76 ICES SCIENTIFIC REPORTS RAPPORTS SCIENTIFIQUES DU CIEM ICES INTERNATIONAL COUNCIL FOR THE EXPLORATION OF THE SEA CIEM CONSEIL INTERNATIONAL POUR L’EXPLORATION DE LA MER International Council for the Exploration of the Sea Conseil International pour l’Exploration de la Mer H.C. Andersens Boulevard 44-46 DK-1553 Copenhagen V Denmark Telephone (+45) 33 38 67 00 Telefax (+45) 33 93 42 15 www.ices.dk [email protected] The material in this report may be reused for non-commercial purposes using the recommended cita- tion. ICES may only grant usage rights of information, data, images, graphs, etc. of which it has owner- ship. For other third-party material cited in this report, you must contact the original copyright holder for permission. For citation of datasets or use of data to be included in other databases, please refer to the latest ICES data policy on ICES website. All extracts must be acknowledged. For other reproduction requests please contact the General Secretary. This document is the product of an expert group under the auspices of the International Council for the Exploration of the Sea and does not necessarily represent the view of the Council. ISSN number: 2618-1371 I © 2020 International Council for the Exploration of the Sea ICES Scientific Reports Volume 2 | Issue 76 WORKSHOP ON THE DISTRIBUTION AND BYCATCH MANAGEMENT OP- TIONS OF LISTED DEEP-SEA SHARK SPECIES (WKSHARK6) Recommended format for purpose of citation: ICES. 2020. Workshop on the distribution and bycatch management options of listed deep-sea shark species (WKSHARK6). -

Case Reports for Species & Habitats on the Initial Draft
OSPAR Commission, 2008: Case reports for the OSPAR List of Threatened and/or Declining Species and Habitats ___________________________________________________________________________________________________ Nomination is likely of regional importance, but not at species Centroscymnus coelolepis, Portuguese level. Dogfish Rarity C. coelolepis Portuguese Dogfish Centroscymnus coelolepis is not rare, is becoming increasingly (Barbosa du Bocage & Brito Capello, 1864) scarce in the northern part of the OSPAR Area. Sensitivity C. coelolepis is considered to be very sensitive to depletion by fisheries because of the severely limiting life history characteristics, particularly a low reproductive output, that are common to this and other deepwater elasmobranch species. These Geographical extent characteristics result in a very low resistance to depletion by fisheries. It is ovoviviparous, giving • OSPAR Regions: I, (II, III), IV, V birth to litters of 13 to 29 young, born at 27-31 cm in length. Though age, growth and gestation period • Biogeographic zones: are not yet known, these are likely to be similar to 8,10,11,12,13,14,15,16,17,18,19 that of related species, with very slow growth, late • Region & Biogeographic zones specified for maturity, long intervals between litters, and extreme longevity. All reproductive stages, including mature decline and/or threat: as above and pregnant females, occur together in the OSPAR Widely distributed in the Atlantic, Indian Ocean and Area, but the largest mature females are found in Western Pacific (see Figure 1). It inhabits slightly shallower water, where they are more likely continental and insular slopes and abyssal plains, to be targeted by longline and gillnet fisheries; on or near the bottom at depths of 270-3,675 m, at exploitation of this reproductively-active sector of temperatures of 5-13°C (this is one of the deepest- the population is particularly damaging to the stock. -

And Their Functional, Ecological, and Evolutionary Implications
DePaul University Via Sapientiae College of Science and Health Theses and Dissertations College of Science and Health Spring 6-14-2019 Body Forms in Sharks (Chondrichthyes: Elasmobranchii), and Their Functional, Ecological, and Evolutionary Implications Phillip C. Sternes DePaul University, [email protected] Follow this and additional works at: https://via.library.depaul.edu/csh_etd Part of the Biology Commons Recommended Citation Sternes, Phillip C., "Body Forms in Sharks (Chondrichthyes: Elasmobranchii), and Their Functional, Ecological, and Evolutionary Implications" (2019). College of Science and Health Theses and Dissertations. 327. https://via.library.depaul.edu/csh_etd/327 This Thesis is brought to you for free and open access by the College of Science and Health at Via Sapientiae. It has been accepted for inclusion in College of Science and Health Theses and Dissertations by an authorized administrator of Via Sapientiae. For more information, please contact [email protected]. Body Forms in Sharks (Chondrichthyes: Elasmobranchii), and Their Functional, Ecological, and Evolutionary Implications A Thesis Presented in Partial Fulfilment of the Requirements for the Degree of Master of Science June 2019 By Phillip C. Sternes Department of Biological Sciences College of Science and Health DePaul University Chicago, Illinois Table of Contents Table of Contents.............................................................................................................................ii List of Tables..................................................................................................................................iv -

Identification Accuracy of Six Species of Deepsea Sharks Sampled at Sea by MPI Observers, October 2016 to December 2017
Identification accuracy of six species of deepsea sharks sampled at sea by MPI observers, October 2016 to December 2017. New Zealand Aquatic Environment and Biodiversity Report No. 203 P.J. McMillan J. Sutherland O. Anderson ISSN 1179-6480 (online) ISBN 978-1-77665-971-5 (online) September 2018 Requests for further copies should be directed to: Publications Logistics Officer Ministry for Primary Industries PO Box 2526 WELLINGTON 6140 Email: [email protected] Telephone: 0800 00 83 33 Facsimile: 04-894 0300 This publication is also available on the Ministry for Primary Industries websites at: http://www.mpi.govt.nz/news-and-resources/publications http://fs.fish.govt.nz go to Document library/Research reports © Crown Copyright – Fisheries New Zealand. Table of Contents EXECUTIVE SUMMARY .................................................................................................................... 1 1. INTRODUCTION .......................................................................................................................... 2 2.1 Sampling design ...................................................................................................................... 3 2.2 Observer sampling .................................................................................................................. 3 2.3 Confirmation of observer identification from observer photographs ...................................... 4 2.4 Confirmation of observer identification from DNA barcoding .............................................. 4 2.5 -

© Iccat, 2007
A2.2 ICCAT Species Codes APPENDIX 2.2: SPECIES CODES Y ello wfin tuna Codes used to identify the ICCAT tuna and tuna-like species as well as by-catch species Atún blanco Tuna and tuna-like species G e r m o n Numerical Alphabetical Scientific Name English SkipjackFra tunancais EspañolR a b i l 1 BFT Thunnus thynnus Northern bluefin tuna Thon rouge du Nord Atún común (Cimarrón) 2 SBF Thunnus maccoyii Southern bluefin tuna Thon rouge du Sud Atún del Sur 3 YFT Thunnus albacares erocablA T hazard-bâtard L i s t a d o 4 ALB Thunnus alalunga erocablA Plain bonito 5 BET Thunnus obesus Bigeye tuna Thon obèse(=Patudo)P a l o m e t tPatudo e 6 BLF Thunnus atlanticus Blackfin tuna Thon à nageoires noires Atún des aletas negras 7 LTA Euthynnus alletteratus Little tunny(=Atl.black skipjack) Thonine commune BacoretaT a s a r t e 8 SKJ Katsuwonus pelamis WBlack a h o o m arlinoatsiL M akaire noir 9 BON Sarda sarda Atlantic bonito Bonite à dos rayé Bonito del AtlánticoA guja negra P e t o 10 FRI Auxis thazard Frigate tuna Auxide Melva 11 BOP Orcynopsis unicolor 12 WAH Acanthocybium solandri Pez espada 13 SSM Scomberomorus maculatus Atlantic SpanishS w mackerel o r d f i s hTh azard atlantique Carite atlántico 14 KGM Scomberomorus cavalla King mackerel Thazard Ebarr sé p a d o n Carite lucio 15 SAI Istiophorus albicans Atlantic sailfish Voilier de l'Atlantique Pez vela del Atlántico 16 BLM Makaira indica 17 BUM Makaira nigricans Atlantic blue marlin Makaire bleu de l'Atlantique Aguja azul del Atlántico 18 WHM Tetrapturus albidus Atlantic white marlin Makaire blanc de l'Atlantique Aguja blanca del Atlántico 28 19 SWO Xiphias gladius 3 20 SPF Tetrapturus pfluegeri Longbill spearfish Makaire bécune Aguja picuda 284 ICCAT MANUAL, 1st Edition (January 2010) 21 TUN Thunnini sanuT ien sédinohT acn senutA pen 23 YOU gnuoY sanut senueJ sédinoht senutA senevój 24 BIL Istiophoridae Marlins,sailfishes,etc. -

North Atlantic Sharks Relevant to Fisheries Management a Pocket Guide Fao
NORTH ATLANTIC SHARKS RELEVANT TO FISHERIES MANAGEMENT A POCKET GUIDE FAO. North Atlantic Sharks Relevant to Fisheries Management. A Pocket Guide. Rome, FAO. 2012. 88 cards. For feedback and questions contact: FishFinder Programme, Marine and Inland Fisheries Service (FIRF), Food and Agriculture Organization of the United Nations, Viale delle Terme di Caracalla, 00153 Rome, Italy. [email protected] Programme Manager: Johanne Fischer, FAO Rome, Italy Author: Dave Ebert, Moss Landing Marine Laboratories, Moss Landing, USA Colour illustrations and cover: Emanuela D’Antoni, FAO Rome, Italy Scientific and technical revisers: Nicoletta De Angelis, Edoardo Mostarda, FAO Rome, Italy Digitization of distribution maps: Fabio Carocci, FAO Rome, Italy Page composition: Edoardo Mostarda, FAO Rome, Italy Produced with support of the EU. Reprint: August 2013 Thedesignations employed and the presentation of material in this information product do not imply the expression of any opinion whatsoever on the part of the Food and Agriculture Organization of the United Nations (FAO) concerning the legal or development status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. The mention of specific companies or products of manufacturers, whether or not these have been patented, does not imply that these have been endorsed or recommended by FAO in preference to others of a similar nature that are not mentioned. The views expressed in this information product are those of the author(s) and do not necessarily reflect the views or policies of FAO. ISBN 978-92-5-107366-7 (print) E-ISBN 978-92-5-107884-6 (PDF) ©FAO 2012 FAO encourages the use, reproduction and dissemination of material in this information product.