Novelties in Rapatea (Rapateaceae) from Colombia Gerardo A
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Catalogue of the Amphibians of Venezuela: Illustrated and Annotated Species List, Distribution, and Conservation 1,2César L
Mannophryne vulcano, Male carrying tadpoles. El Ávila (Parque Nacional Guairarepano), Distrito Federal. Photo: Jose Vieira. We want to dedicate this work to some outstanding individuals who encouraged us, directly or indirectly, and are no longer with us. They were colleagues and close friends, and their friendship will remain for years to come. César Molina Rodríguez (1960–2015) Erik Arrieta Márquez (1978–2008) Jose Ayarzagüena Sanz (1952–2011) Saúl Gutiérrez Eljuri (1960–2012) Juan Rivero (1923–2014) Luis Scott (1948–2011) Marco Natera Mumaw (1972–2010) Official journal website: Amphibian & Reptile Conservation amphibian-reptile-conservation.org 13(1) [Special Section]: 1–198 (e180). Catalogue of the amphibians of Venezuela: Illustrated and annotated species list, distribution, and conservation 1,2César L. Barrio-Amorós, 3,4Fernando J. M. Rojas-Runjaic, and 5J. Celsa Señaris 1Fundación AndígenA, Apartado Postal 210, Mérida, VENEZUELA 2Current address: Doc Frog Expeditions, Uvita de Osa, COSTA RICA 3Fundación La Salle de Ciencias Naturales, Museo de Historia Natural La Salle, Apartado Postal 1930, Caracas 1010-A, VENEZUELA 4Current address: Pontifícia Universidade Católica do Río Grande do Sul (PUCRS), Laboratório de Sistemática de Vertebrados, Av. Ipiranga 6681, Porto Alegre, RS 90619–900, BRAZIL 5Instituto Venezolano de Investigaciones Científicas, Altos de Pipe, apartado 20632, Caracas 1020, VENEZUELA Abstract.—Presented is an annotated checklist of the amphibians of Venezuela, current as of December 2018. The last comprehensive list (Barrio-Amorós 2009c) included a total of 333 species, while the current catalogue lists 387 species (370 anurans, 10 caecilians, and seven salamanders), including 28 species not yet described or properly identified. Fifty species and four genera are added to the previous list, 25 species are deleted, and 47 experienced nomenclatural changes. -

Amazon Alive: a Decade of Discoveries 1999-2009
Amazon Alive! A decade of discovery 1999-2009 The Amazon is the planet’s largest rainforest and river basin. It supports countless thousands of species, as well as 30 million people. © Brent Stirton / Getty Images / WWF-UK © Brent Stirton / Getty Images The Amazon is the largest rainforest on Earth. It’s famed for its unrivalled biological diversity, with wildlife that includes jaguars, river dolphins, manatees, giant otters, capybaras, harpy eagles, anacondas and piranhas. The many unique habitats in this globally significant region conceal a wealth of hidden species, which scientists continue to discover at an incredible rate. Between 1999 and 2009, at least 1,200 new species of plants and vertebrates have been discovered in the Amazon biome (see page 6 for a map showing the extent of the region that this spans). The new species include 637 plants, 257 fish, 216 amphibians, 55 reptiles, 16 birds and 39 mammals. In addition, thousands of new invertebrate species have been uncovered. Owing to the sheer number of the latter, these are not covered in detail by this report. This report has tried to be comprehensive in its listing of new plants and vertebrates described from the Amazon biome in the last decade. But for the largest groups of life on Earth, such as invertebrates, such lists do not exist – so the number of new species presented here is no doubt an underestimate. Cover image: Ranitomeya benedicta, new poison frog species © Evan Twomey amazon alive! i a decade of discovery 1999-2009 1 Ahmed Djoghlaf, Executive Secretary, Foreword Convention on Biological Diversity The vital importance of the Amazon rainforest is very basic work on the natural history of the well known. -

Gondwanan Origin of Major Monocot Groups Inferred from Dispersal-Vicariance Analysis Kåre Bremer Uppsala University
Aliso: A Journal of Systematic and Evolutionary Botany Volume 22 | Issue 1 Article 3 2006 Gondwanan Origin of Major Monocot Groups Inferred from Dispersal-Vicariance Analysis Kåre Bremer Uppsala University Thomas Janssen Muséum National d'Histoire Naturelle Follow this and additional works at: http://scholarship.claremont.edu/aliso Part of the Botany Commons Recommended Citation Bremer, Kåre and Janssen, Thomas (2006) "Gondwanan Origin of Major Monocot Groups Inferred from Dispersal-Vicariance Analysis," Aliso: A Journal of Systematic and Evolutionary Botany: Vol. 22: Iss. 1, Article 3. Available at: http://scholarship.claremont.edu/aliso/vol22/iss1/3 Aliso 22, pp. 22-27 © 2006, Rancho Santa Ana Botanic Garden GONDWANAN ORIGIN OF MAJOR MONO COT GROUPS INFERRED FROM DISPERSAL-VICARIANCE ANALYSIS KARE BREMERl.3 AND THOMAS JANSSEN2 lDepartment of Systematic Botany, Evolutionary Biology Centre, Norbyvagen l8D, SE-752 36 Uppsala, Sweden; 2Museum National d'Histoire Naturelle, Departement de Systematique et Evolution, USM 0602: Taxonomie et collections, 16 rue Buffon, 75005 Paris, France 3Corresponding author ([email protected]) ABSTRACT Historical biogeography of major monocot groups was investigated by biogeographical analysis of a dated phylogeny including 79 of the 81 monocot families using the Angiosperm Phylogeny Group II (APG II) classification. Five major areas were used to describe the family distributions: Eurasia, North America, South America, Africa including Madagascar, and Australasia including New Guinea, New Caledonia, and New Zealand. In order to investigate the possible correspondence with continental breakup, the tree with its terminal distributions was fitted to the geological area cladogram «Eurasia, North America), (Africa, (South America, Australasia») and to alternative area cladograms using the TreeFitter program. -

FRESH-WATER SHRIMPS (CRUSTACEA, DECAPODA, NATANTIA) of the ORINOCO BASIN and the VENEZUELAN GUAYANA Gilberto Rodriguez the Guaya
JOURNAL OF CRUSTACEAN BIOLOGY, 2(3): 378-391, 1982 FRESH-WATER SHRIMPS (CRUSTACEA, DECAPODA, NATANTIA) OF THE ORINOCO BASIN AND THE VENEZUELAN GUAYANA Gilberto Rodriguez ABSTRACT Shrimps of the families Sergestidae and Palaemonidae collected in the Orinoco basin, the upper Cuyuni River, and the upper and lower Rio Negro, are dealt with in this paper. New records and comments are given for Acetes paraguayensis, Macrobrachium amazonicum, M. brasiliense, M. jelskii, M. nattered, M. surinamicum, and Palaemonetes carteri. Two new palaemonids are described: Macrobrachium cortezi, a form related to M. nattereri, from several localities in the Orinoco River and upper Rio Negro, and M. aracamuni, from an altitude of 680 m in the Cerro Aracamuni in the drainage area of the upper Rio Negro. Another previously undescribed species of Macrobrachium is recorded but not named due to the lack of mature males. The Guayana highland is an ancient land mass extending from the Amazon River to the Atlantic coast of South America and includes the Guianas and parts of Venezuela and Brazil. The Venezuelan Guayana comprises 41,300 km2 of territory, mostly above 400 m that separate the Orinoco from the Amazon basin and forms a formidable barrier to the dispersion of the fresh-water fauna of the lowlands. The hydrology of the zone is defined by the Orinoco River that bounds the area to the west and north and its tributaries that generally flow north or northwesterly. A smaller portion to the east is drained by the Cuyuni River. The Orinoco and the Amazon basins are connected through the Brazo Casiquiare, while the inundated savannah of Rupununi allows intermittent connections be tween the Branco and the Esequibo Rivers (Lowe-McConnell, 1964). -
Diversity and Evolution of Monocots
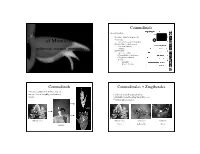
Commelinids 4 main groups: Diversity and Evolution • Acorales - sister to all monocots • Alismatids of Monocots – inc. Aroids - jack in the pulpit • Lilioids (lilies, orchids, yams) – non-monophyletic . spiderworts, bananas, pineapples . – petaloid • Commelinids – Arecales – palms – Commelinales – spiderwort – Zingiberales –banana – Poales – pineapple – grasses & sedges Commelinids Commelinales + Zingiberales • theme: reduction of flower, loss of nectar, loss of zoophily, evolution of • 2 closely related tropical orders bracts • primarily nectar bearing but with losses • bracted inflorescences grass pickeral weed pickeral weed spiderwort heliconia nectar pollen only bracts rapatead bromeliad Commelinaceae - spiderwort Commelinaceae - spiderwort Family of small herbs with succulent stems, stems jointed; leaves sheathing. Family does not produce Inflorescence often bracted nectar, but showy flowers for insect pollen gathering. Rhoeo - Moses in a cradle Commelina erecta - Erect dayflower Tradescantia ohiensis - spiderwort Tradescantia ohiensis - spiderwort Commelinaceae - spiderwort Commelinaceae - spiderwort Flowers actinomorphic or • species rich in pantropics, CA 3 CO 3 A 6 G (3) zygomorphic especially Africa • floral diversity is enormous Commelina communis - day flower Tradescantia ohiensis - spiderwort Pontederiaceae - pickerel weed Pontederiaceae - pickerel weed Aquatic family of emergents or floaters. Pickerel weed has glossy heart-shaped leaves, Water hyacinth (Eichhornia) from superficially like Sagittaria but without net venation. -

Evolutionary History of Floral Key Innovations in Angiosperms Elisabeth Reyes
Evolutionary history of floral key innovations in angiosperms Elisabeth Reyes To cite this version: Elisabeth Reyes. Evolutionary history of floral key innovations in angiosperms. Botanics. Université Paris Saclay (COmUE), 2016. English. NNT : 2016SACLS489. tel-01443353 HAL Id: tel-01443353 https://tel.archives-ouvertes.fr/tel-01443353 Submitted on 23 Jan 2017 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. NNT : 2016SACLS489 THESE DE DOCTORAT DE L’UNIVERSITE PARIS-SACLAY, préparée à l’Université Paris-Sud ÉCOLE DOCTORALE N° 567 Sciences du Végétal : du Gène à l’Ecosystème Spécialité de Doctorat : Biologie Par Mme Elisabeth Reyes Evolutionary history of floral key innovations in angiosperms Thèse présentée et soutenue à Orsay, le 13 décembre 2016 : Composition du Jury : M. Ronse de Craene, Louis Directeur de recherche aux Jardins Rapporteur Botaniques Royaux d’Édimbourg M. Forest, Félix Directeur de recherche aux Jardins Rapporteur Botaniques Royaux de Kew Mme. Damerval, Catherine Directrice de recherche au Moulon Président du jury M. Lowry, Porter Curateur en chef aux Jardins Examinateur Botaniques du Missouri M. Haevermans, Thomas Maître de conférences au MNHN Examinateur Mme. Nadot, Sophie Professeur à l’Université Paris-Sud Directeur de thèse M. -

Zi[ EN BIODIVERSIDAD)? 11I
_ ---------- -i _l_ - ________ _ _ .. ~~~_._.G=.:_.__ _ _ ._.._,_,___,_____ _,_,o_ssw__ r____I_____.-- ,.................. ,,. 15_ j s - -- ,,.nn......................................... == -- -_: _: . -. - . = -- LO NI~~~- Public Disclosure Authorized CIP)~~ ~ ~~- = I 7 i s s s Public Disclosure Authorized - i, z 2. L ~LLj t1~ t !- (9~ ~ ~ ~ ~ ~ ~ ~ ~~~~~~~~~~L Public Disclosure Authorized 0E 0 . _ - . _ _ - . r~ ~ ~ ~ .. Public Disclosure Authorized . I''U' "71111 ;:01i11:Ii 1^/]N: 1 I 1 HO1riON INVESTMENTS: I diversity Funding in H i J the Caribbean I ( Z t rt .I 'I ussell, L. Cornwell and E. Fajer zI[ EN BIODIVERSIDAD)? 11i. lEvwili I liento para la Biodiversidad ri c I 1:i na y el Caribe (: ( Linv c ) 1 Russell, L. Cornwell y E. Fajer 35111 Biodiversity Support Program Washington, DC 'l t 1 DII" World Bank -~W .~ ~ ~ ~ ~ ~ ~ ~~~~~. IA11I.E (! ) iTi NTrs TABLA DE CONTENIDOS Ackrio' v eil I ntsri 5 Reconocimientos 5 Exerut ve r via ryt 7 Resumen Ejecutivo 7 I i -,)du I c- to Introduccion Io 're\ c:l', ( ' w tion Funri v k ;essn mnt 12 Evaluaciones Previas sobre Financiamiento Bloc vn r! j r dme Asscs i 1 i i )r LA 13 para la Conservaci6n 12 Evaluacion del Financiamiento para la Mle t iod i 14 Biodiversidad en LAC 13 Metodos '4 Info nia: ( ^ llected 14 Encuesta 14 IPot( ] la ii m-sof Errot 15 Informacion Recolectada 14 Re5zu Its et id t ssicn 1 Fuentes Potenciales de Error 15 Gen r:il t .I s i6 Resultados y Discusi6n 16 Fun itig 1 nor Type 20 Resultados Generales i6 Funi itig ) r jecl Caategoi 21 Financiamiento por Tipo de Donante 20 -

GENOME EVOLUTION in MONOCOTS a Dissertation
GENOME EVOLUTION IN MONOCOTS A Dissertation Presented to The Faculty of the Graduate School At the University of Missouri In Partial Fulfillment Of the Requirements for the Degree Doctor of Philosophy By Kate L. Hertweck Dr. J. Chris Pires, Dissertation Advisor JULY 2011 The undersigned, appointed by the dean of the Graduate School, have examined the dissertation entitled GENOME EVOLUTION IN MONOCOTS Presented by Kate L. Hertweck A candidate for the degree of Doctor of Philosophy And hereby certify that, in their opinion, it is worthy of acceptance. Dr. J. Chris Pires Dr. Lori Eggert Dr. Candace Galen Dr. Rose‐Marie Muzika ACKNOWLEDGEMENTS I am indebted to many people for their assistance during the course of my graduate education. I would not have derived such a keen understanding of the learning process without the tutelage of Dr. Sandi Abell. Members of the Pires lab provided prolific support in improving lab techniques, computational analysis, greenhouse maintenance, and writing support. Team Monocot, including Dr. Mike Kinney, Dr. Roxi Steele, and Erica Wheeler were particularly helpful, but other lab members working on Brassicaceae (Dr. Zhiyong Xiong, Dr. Maqsood Rehman, Pat Edger, Tatiana Arias, Dustin Mayfield) all provided vital support as well. I am also grateful for the support of a high school student, Cady Anderson, and an undergraduate, Tori Docktor, for their assistance in laboratory procedures. Many people, scientist and otherwise, helped with field collections: Dr. Travis Columbus, Hester Bell, Doug and Judy McGoon, Julie Ketner, Katy Klymus, and William Alexander. Many thanks to Barb Sonderman for taking care of my greenhouse collection of many odd plants brought back from the field. -

Information Sheet on Ramsar Wetlands (RIS) – 2009-2012 Version Available for Download From
Information Sheet on Ramsar Wetlands (RIS) – 2009-2012 version Available for download from http://www.ramsar.org/ris/key_ris_index.htm. Categories approved by Recommendation 4.7 (1990), as amended by Resolution VIII.13 of the 8th Conference of the Contracting Parties (2002) and Resolutions IX.1 Annex B, IX.6, IX.21 and IX. 22 of the 9th Conference of the Contracting Parties (2005). Notes for compilers: 1. The RIS should be completed in accordance with the attached Explanatory Notes and Guidelines for completing the Information Sheet on Ramsar Wetlands. Compilers are strongly advised to read this guidance before filling in the RIS. 2. Further information and guidance in support of Ramsar site designations are provided in the Strategic Framework and guidelines for the future development of the List of Wetlands of International Importance (Ramsar Wise Use Handbook 14, 3rd edition). A 4th edition of the Handbook is in preparation and will be available in 2009. 3. Once completed, the RIS (and accompanying map(s)) should be submitted to the Ramsar Secretariat. Compilers should provide an electronic (MS Word) copy of the RIS and, where possible, digital copies of all maps. 1. Name and address of the compiler of this form: FOR OFFICE USE ONLY. DD MM YY Beatriz de Aquino Ribeiro - Bióloga - Analista Ambiental / [email protected], (95) Designation date Site Reference Number 99136-0940. Antonio Lisboa - Geógrafo - MSc. Biogeografia - Analista Ambiental / [email protected], (95) 99137-1192. Instituto Chico Mendes de Conservação da Biodiversidade - ICMBio Rua Alfredo Cruz, 283, Centro, Boa Vista -RR. CEP: 69.301-140 2. -

Lowland Rainforests
Glime, J. M. 2019. Tropics: Lowland Rainforests. Chapt. 8-7. In: Glime, J. M. Bryophyte Ecology. Volume 4. Habitat and Role. 8-7-1 Ebook sponsored by Michigan Technological University and the International Association of Bryologists. Last updated 22 July 2020 and available at <http://digitalcommons.mtu.edu/bryophyte-ecology4/>. CHAPTER 8-7 TROPICS: LOWLAND RAINFORESTS TABLE OF CONTENTS Lowland Rainforests ........................................................................................................................................... 8-7-2 Amazonia Lowlands ............................................................................................................................................ 8-7-7 Terra Firme ................................................................................................................................................ 8-7-11 Dense Forest ....................................................................................................................................... 8-7-14 Open Forest without Palms ................................................................................................................. 8-7-14 Open Forest with Palms ...................................................................................................................... 8-7-14 Liana Forest ........................................................................................................................................ 8-7-16 Dry Forest .......................................................................................................................................... -

Pollen Morphology of Rapateaceae Sherwin Carlquist Claremont Graduate School
Aliso: A Journal of Systematic and Evolutionary Botany Volume 5 | Issue 1 Article 7 1961 Pollen Morphology of Rapateaceae Sherwin Carlquist Claremont Graduate School Follow this and additional works at: http://scholarship.claremont.edu/aliso Part of the Botany Commons Recommended Citation Carlquist, Sherwin (1961) "Pollen Morphology of Rapateaceae," Aliso: A Journal of Systematic and Evolutionary Botany: Vol. 5: Iss. 1, Article 7. Available at: http://scholarship.claremont.edu/aliso/vol5/iss1/7 ALISO VoL. 5, No. 1, pp. 39-66 MAY 15, 1961 POLLEN MORPHOLOGY OF RAPATEACEAE SHERWIN CARLQUIST1 Claremont Graduate School, Claremont, California INTRODUCTION Several circumstances prompt the presentation of studies on pollen morphology of Rapa teaceae at this time. First, recent explorations in the Guayana Highland region of northern South America have amassed excellent collections in this interesting monocotyledonous family. The family is restricted to South America with the exception of a monotypic genus in western tropical Africa (Maguire et al., 1958). Although there is no reason to believe that all new species in the family have been described, the remarkable collections of material certainly do furnish an extensive basis for pollen study which has not been available previ ously. Of great significance in this regard are the collections of Maguire and his associates at the New York Botanical Garden. Many of these collections included liquid-preserved portions. A second reason for presentation of pollen studies is the excellent condition in which pollen-bearing material of many species was preserved. Because the delicate nature of pollen grains of Rapateaceae, as described below, makes preparation of pollen-grain slides from dried material often unsatisfactory, liquid-preserved material is especially im portant. -

Minería, Guerrilla Y Enfermedades: El Legado De La Revolución a Los Indígenas De La Reserva De Biosfera Alto Orinoco Casiquiare, Amazonas Venezolano
Minería, guerrilla y enfermedades: el legado de la revolución a los indígenas de la Reserva de Biosfera Alto Orinoco Casiquiare, Amazonas Venezolano Informe de actualización: agosto 2019 - julio 2020 Caracas, Venezuela Julio 2020 SOSORINOCO SOSORINOCO 2020 Página | 1 “La Comisión ha manifestado que… comunidades del pueblo Yanomami, en cuyos territorios se practica la minería ilegal, habrían sufrido agresiones de mineros y violaciones a mujeres”. Comisión Interamericana de Derechos Humanos. Informe Anual 2019. Washington, DC., febrero 24, 2020. “Hoy existe mayor riesgo para los Yanomami que en 1993. Una muestra del incumplimiento del acuerdo amistoso”. Provea. 27 años después de la masacre de Haximú indígenas yanomami denuncian presencia de mineros y complicidad de autoridades. Junio 14, 2020. “Los Yanomami son una parte esencial de la herencia diversa del mundo. Ayudan a contar la historia de todos los pueblos de la Amazonía. Permitir la disminución o destrucción de esta invaluable comunidad sería un terrible error”. Alex Barker & Edward Liebow. American Anthropological Association. Arlington, VA, julio 22, 2019 RBAOC. Informe de actualización (agosto 2019 - julio 2020) SOSORINOCO 2020 Página | 2 Contenido Lista De Figuras ....................................................................................................... 2 Introducción ............................................................................................................. 3 Capítulo I ................................................................................................................