Introduction
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A J U N T a M E N T D' a L C a N
Núm. 6/2011 A J U N T A M E N T D’ A L C A N A R ACTA DEL PLE DE DATA 03/05/2011 Alcanar, tres de maig de l’any dos mil onze . Essent les 12:20 hores es reuneixen al Saló de Sessions d'aquest Ajuntament a l'objecte de celebrar sessió extraordinària de 1ª convocatòria, sota la Presidència del Sr. Alcalde, ALFONS MONTSERRAT ESTELLER, i amb l'assistència de l’Interventor de fos, en SEBASTIÀ BATISTE MIRALLES, la Secretària Acctal. na Ma DOLORES RÓO GARCÍA com a fefaent; i amb l’assistència dels regidors següents: En JOSEP Ma SANCHO SUBIRATS Regidor Na Ma MERCÈ FIBLA NOLLA Regidora En JOSEP MANEL MARTÍ MONTESINOS Regidor Na Ma ISABEL SELMA FONTET Regidora En JOSEP BORT ESTELLER Regidor En RICARD BORT FIBLA Regidor En DIEGO ALCOBA FERNÁNDEZ Regidor En JOAQUIM LLAGOSTERA CASTELL Regidor En JOSEP BELTRAN VALLS Regidor Na Ma JOSÉ SUBIRATS REVERTER Regidora En JOAN MATAMOROS GUIMERÀ Regidor No assisteix i s’excusa l'absència: En JOAN BTA. BELTRAN QUERALT Regidor Obert l'acte de la Presidència, són tractats i debatuts els assumptes inclosos en l'Ordre del Dia, prenent-se els següents ACORDS: 1.- RECTIFICACIÓ DEL PROGRAMA DE FESTES I D’ACTES TAURINS DE LES FESTES DE MAIG 2011.- Per l’Alcaldia es concedeix la paraula al regidor de Festes , en Josep Bort Esteller (CIU-PA i LC), el qual dóna compte de la proposta de rectificació del programa de festes i d’actes taurins de les Festes de Maig, derivat de l’obligació legal de la Llei 34/2010, de l’1 d’octubre, de regulació de les festes tradicionals amb bous, pel que fa a la modificació del recorregut del bou capllaçat, per motius d’unes obres que s’estàn realitzant, així com per la inclusió d’uns bous salvatges. -

Morfologia Històrica Del Territorium De Tarraco En Època
ANNEX IV – Inventari i mapa de localització dels llocs de culte cristià i dels cementiris del Camp de Tarragona (Mapa 11). ANNEX IV – INVENTARI I MAPA DE LOCALITZACIÓ DELS LLOCS DE CULTE CRISTIÀ I DELS CEMENTIRIS DEL CAMP DE TARRAGONA. A continuació presentem l’inventari amb el seu corresponent mapa d’ubicació (amb una escala original 1:50.000) dels llocs de culte cristià (creus, esglèsies, ermites, oratoris, etc...) i d’enterrament que hem pogut constatar al Camp de Tarragona (Mapa 10)1. Es tracta d’elements molt interessants que poden atorgar-nos valuoses informacions sobre l’antiga morfologia del territori que ens ocupa, doncs la seva especial importància ha afavorit la seva conservació. Sovint han conservat un emplaçament original ben antic o bé s’han superposat a indrets d’una significació anterior. En el cas concret de la centuriació, és ben conegut el fenomen de cristianització d’elements de la xarxa cadastral (fites convertides en creus de terme, altars de camí esdevinguts ermites, encreuaments ocupats per esglesioles, etc...) (veure III Part, capítol 5, apartat 5.3.3.)2. En conseqüència, el material que seguidament exposarem (localitzat, fonamentalment, treballant sobre materials cartogràfics a escala 1:25.000 i 1:50.000) ha proporcionat tot un seguit de dades que han estat molt importants a l’hora de realitzar l’anàlisi arqueomorfològica desenvolupada al Capítol 5 de la Part III. 1.- TARRAGONÈS. 1.1.- Altafulla. 1.1.1.- Ermita de Sant Antoni de Pàdua. 1.1.2.- Cementiri d’Altafulla. 1.2.- El Catllar de Gaià. 1.2.1.- Ermita de Sant Ramon. -

ASCO Proceedings
BREAST CANCER—TRIPLE-NEGATIVE/CYTOTOXICS/LOCAL THERAPY LBA1000 Oral Abstract Session, Tue, 9:45 AM-12:45 PM NSABP B-38: Definitive analysis of a randomized adjuvant trial comparing dose- dense (DD) AC followed by paclitaxel (P) plus gemcitabine (G) with DD AC followed by P and with docetaxel, doxorubicin, and cyclophosphamide (TAC) in women with operable, node-positive breast cancer. Sandra M. Swain, Gong Tang, Charles E. Geyer, Priya Rastogi, James Norman Atkins, Paul P. Donnellan, Louis Fehrenbacher, Catherine A. Azar, Andre Robidoux, Jonathan Polikoff, Adam Brufsky, David D. Biggs, Edward A. Levine, John L. Zapas, Louise Provencher, Edith A. Perez, Soonmyung Paik, Joseph P. Costantino, Eleftherios P. Mamounas, Norman Wolmark; National Surgical Adjuvant Breast and Bowel Project and Washington Cancer Institute, MedStar Washington Hospital Center, Washington, DC; NSABP Biostatistical Center and University of Pittsburgh Graduate School of Public Health, Department of Biostatistics, Pittsburgh, PA; National Surgical Adjuvant Breast and Bowel Project and University of Texas, Southwestern Medical Center, Dallas, TX; National Surgical Adjuvant Breast and Bowel Project and University of Pittsburgh Cancer Institute, Pittsburgh, PA; National Surgical Adjuvant Breast and Bowel Project and SCCC-CCOP, Goldboro, NC; All Ireland Cooperative Oncology Research Group and University Hospital Galway, Galway, Ireland; National Surgical Adjuvant Breast and Bowel Project and Kaiser Permanente Northern California, Vallejo, CA; National Surgical Adjuvant Breast and Bowel Project and Kaiser Permanente, Denver, CO; National Surgical Adjuvant Breast and Bowel Project and Centre Hospitalier de l’Universite de Montreal, Montreal, QC, Canada; National Surgical Breast and Bowel Project and Kaiser Permanente Southern California, San Diego, CA; National Surgical Adjuvant Breast and Bowel Project and University of Pittsburgh, Magee-Womens Hospital, Pittsburgh, PA; National Surgical Adjuvant Breast and Bowel Project and Helen F. -
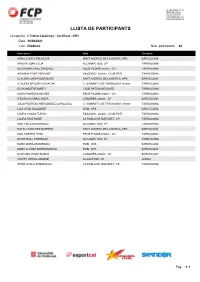
Inscripcions Dimecres 30/6
LLISTA DE PARTICIPANTS Competició: I-Trofeu Catalunya - Certificat - GR3 Data: 30/06/2021 Lloc: Riudoms Núm. participants: 22 Participant Club Territorial AINA LLORCA PALACIOS SANT ANDREU DE LA BARCA, APA BARCELONA AINOHA JUAN CLUA ALCANAR 1003, CP TARRAGONA ALICIA MARTÍNEZ SÁNCHEZ REUS PLOMS-artístic-, CN TARRAGONA ARIADNA FONT FERRANT RIUDOMS - artístic-, CLUB PATI TARRAGONA CLAUDIA LARA RODRIGUEZ SANT ANDREU DE LA BARCA, APA BARCELONA CLÀUDIA SEGURA GAVALDÀ C. GIMNÀSTIC DE TARRAGONA -Artístic- TARRAGONA ELGA MESTRE MARTÍ CLUB PATÍ MONTSKATE TARRAGONA HANA PAMIES SANCHEZ REUS PLOMS-artístic-, CN TARRAGONA ITZIAR NAVARRO ARIZA CONGRÉS-artístic-, CP BARCELONA JÚLIA PUERTAS HERNÁNDEZ-CAPALLEJA C. GIMNÀSTIC DE TARRAGONA -Artístic- TARRAGONA LAIA LEON GALOBART RUBI, CPA BARCELONA LAURA CASAS TURÓN RIUDOMS - artístic-, CLUB PATI TARRAGONA LAURA RUIZ MAÑÉ LA POBLA DE MAFUMET, CP TARRAGONA MAR FIBLA DOMINGUEZ ALCANAR 1003, CP TARRAGONA NATALI SANCHEZ MORENO SANT ANDREU DE LA BARCA, APA BARCELONA NOA TORRES LEÓN REUS PLOMS-artístic-, CN TARRAGONA NOOR NOLL FABREGAT ALCANAR 1003, CP TARRAGONA NORA DURA ZAMORANO RUBI, CPA BARCELONA NORA LLANSÀ BARRIONUEVO RUBI, CPA BARCELONA SARA DEL POZO BOSCH CONGRÉS-artístic-, CP BARCELONA VINYET FREIXA ARGEMÍ ALCOLETGE, CP LLEIDA XÈNIA AYALA SAMANIEGO LA POBLA DE MAFUMET, CP TARRAGONA Pàg. 1 / 1 LLISTA DE PARTICIPANTS Competició: I-Trofeu Catalunya - Iniciació A - GR3 Data: 30/06/2021 Lloc: Riudoms Núm. participants: 27 Participant Club Territorial ABRIL CUELLA PEÑA SANT ANDREU DE LA BARCA, APA BARCELONA AINHOA -

Ager Tarraconensis 1 Aspectes Històrics I Marc Natural Historical Aspects and Natural Setting
Ager Tarraconensis 1 Aspectes històrics i marc natural Historical aspects and natural setting Marta Prevosti i Josep Guitart i Duran (directors científics / Scientific Directors) Three chapters in English 16 Institut d’Estudis Catalans Institut Català d’Arqueologia Clàssica Tarragona, 2010 Biblioteca de Catalunya - Dades CIP Ager Tarraconensis. – (Documenta ; 16) Bibliografia. – Conté: 1. Aspectes històrics i marc natural -- 2. El poblament -- 3. Les inscripcions romanes (IRAT). – Text en català, alguns capítols també en anglès ISBN 9788493773434 (o.c.) I. Prevosti, Marta, dir. II. Guitart i Duran, Josep, 1946- dir. III. Gorostidi, Diana IV. Institut d’Estudis Catalans V. Institut Català d’Arqueologia Clàssica VI. Col·lecció: Documenta (Institut Català d’Arqueologia Clàssica) ; 16 1. Arqueologia del paisatge – Catalunya – Camp de Tarragona 2. Excavacions arqueològiques – Catalunya – Camp de Tarragona 3. Camp de Tarragona (Catalunya) – Arqueologia romana 904(467.14) Ager Tarraconensis és un projecte de l’Institut Català d’Arqueologia Clàssica i l’Institut d’Estudis Catalans, amb el finança- ment d’Acesa-Abertis. El projecte s’emmarca dins la línia de recerca de l’ICAC «Arqueologia del paisatge, poblament i terri- tori» i del projecte Forma Orbis Romani de l’Institut d’Estudis Catalans, promogut per la Unió Acadèmica Internacional. Aquesta recerca també s’ha inserit en el marc del projecte del Ministeri de Ciència i Innovació HAR2009-10752: «Interacció i articulació urbs-territorium en el conventus Tarraconensis». © d’aquesta edició, Institut d’Estudis Catalans i Institut Català d’Arqueologia Clàssica (ICAC) Institut Català d’Arqueologia Clàssica Plaça d’en Rovellat, s/n, 43003 Tarragona Telèfon 977 24 91 33 - fax 977 22 44 01 [email protected] - www.icac.net © del text, els autors © de les fotografies i il·lustracions, els autors, llevat que s’indiqui el contrari © de la traducció a l’anglès, Paul Turner (capítols 2 i 5.4.3.) i Encarna Raga Almúdever (capítol 4) © de la foto de la coberta, Arxiu MNAT / M. -

Tarragona in Figures
>> Tarragona in figures 2012 00 01 02 03 04 05 06 07 08 >> Tarragona in figures > Edited by: TARRAGONA CITY HALL Council of Labour, Economic Development and Youth Politics > Work team: Vicenç Alcaraz Santiago Castellà Catalina Jordi, CREP-URV Angel Martorell Montserrat Pascual Juan Manuel Patón > Special collaboration: Tarragona Chamber of Commerce CEPTA (Tarragona Business Confederation) – Department of Studies > Translation: Tarraco Translation > Design and layout: Department Corporate Image and Desktop Publishing >> Index > PRESENTATIONS ................................................................................................... 7 > 00. EXECUTIVE SUMMARY ................................................................................... 10 > 01. A PRIVILEGED LOCATION ................................................................................ 18 > 02. THE SECOND LARGEST ECONOMIC CENTRE OF CATALONIA .......................... 22 > 03. HIGHLY ACCESSIBLE AND INTERNATIONALLY CONNECTED .......................... 28 > 04. HUMAN CAPITAL ............................................................................................. 32 > 05. INDUSTRIAL ACTIVITY, GROWTH AND ENERGY GENERATION ........................ 40 > 06. TOURISM, CULTURE AND ACTIVE COMMERCE ............................................... 46 > 07. QUALITY OF LIFE ............................................................................................ 54 > 08. OLYMPIC CITY ................................................................................................ -

Locals De Participació Per Municipi.Xlsx
Nom vegueria Nom comarca Nom Municipi NOM_LOCAL ADREÇA_LOCAL COD_POSTAL MESES Barcelona Alt Penedès Avinyonet del Penedès Baixos Edifici Ajuntament Pl. de la Vila, 1 08793 2 Barcelona Alt Penedès Cabanyes, les Centre Cultural i Lúdic Carrer Trenta Quatre, 6 08794 1 Barcelona Alt Penedès Castellet i la Gornal Centre Civic la Gornal Rambla, 6 08729 3 Barcelona Alt Penedès Castellví de la Marca Edifici Polivalent Municipal Anselm Clavé 08732 2 Barcelona Alt Penedès Font‐rubí Centre Recreatiu Av. Catalunya, 45 08736 2 Barcelona Alt Penedès Gelida Institut Gelida c. de Joan Pascual i Batlle, 1‐15 08790 6 Barcelona Alt Penedès Granada, la Centre Cívic Av. Catalunya, 70 08792 2 Barcelona Alt Penedès Mediona Casal d'Avis Baixos de l'Aj. C. Dr. Trueta, 10 08773 2 Barcelona Alt Penedès Olèrdola Centre Cívic "La Xarxa" Av. de la Carrerada, 31‐33 08734 3 Barcelona Alt Penedès Olesa de Bonesvalls Sala d'Actes Ajuntament Plaça de la Vila, 1 08795 2 Barcelona Alt Penedès Pacs del Penedès CEIP "El Drac" de Pacs Carrer de les Escoles 08796 1 Barcelona Alt Penedès Pla del Penedès, el EP. Sants Abdó i Senen C. Sants Abdó i Senen, 9 08733 2 Barcelona Alt Penedès Puigdàlber Centre Cívic Av. Catalunya, 8 08797 1 Barcelona Alt Penedès Sant Cugat Sesgarrigues Aula d'Informàtica Centre Cívic C/Ponent 7 08798 1 Barcelona Alt Penedès Sant Llorenç d'Hortons Escola Pau Casals Pau Casals, 12 08791 3 Barcelona Alt Penedès Sant Martí Sarroca Institut de l'Alt Foix av. Verge de Montserrat, 14 08731 2 Barcelona Alt Penedès Sant Martí Sarroca Escola Jaume Balmes C. -

Environmental Impact Assessment
Av. Marià Fortuny 83, 2n 3a 43203, REUS [email protected] www.limonium.org 977 342 069 Presentation LIMONIUM, SOCIETAT d’ACTUACIONS AMBIENTALS, was born as a company on April 1996. It is formed by a pluridisciplinar team of young but experienced professionals, educated in the items of environmental sciences, engineering, biology, land planning and environmental education. Limonium offers its experience to companies, public administrations and individuals, and because of this, two different working teams have been created: environmental consulting and communication and environmental education. Image of ENVIRONMENTAL CONSULTING AREA OF LIMONIUM: studies, projects, and assessments Image of COMMUNICATION and ENVIRONMENTAL EDUCATION TEAM OF LIMONIUM: education for sustainability and environmental concernment, for schools and general public. Limonium spp. Limonium is the latin name of a genus of plants named Sea-Lavanders or Statice in English. They belong to the famíly Plumbaginaceae, being small and generally linked to salty environments (salty marshes, inland and coastal salty soils and first coastal line). They consist morfologically on a basal rosette of leafs, usually fleshy, and long floral stems, with colors that change from white to violet through pink. The essence of genus Limonium defines the philosophy of our company: • Diversity: genus Limonium has more than 100 species in the Iberian Peninsula • Adaptation to local conditions: there is a different Limonium in almost every different cliff or salty marsh. • Adaptation to difficult conditions: Limonium plants always grow in difficult environments, in places that are harsh for other species. • Humility: Limonium are always humble plants, that know their limitations and that only flower when conditions are proper. -

Desplegament Fibra Òptica 2019-2021 Demarcació De Tarragona
Desplegament 2020-2022 demarcació de Tarragona Cristina Campillo i Cruellas – Gencat.cat Jaume Vidal González – Diputació de Tarragona Versió 1 – Gener de 2021 Desplegament 2020 2 Desplegament 2020 (I). Capitals de comarca. El 2020, s’ha fet el desplegament de capitals de comarca, obres promogudes per la Secretaria de Polítiques Digitals (SPD). Llegenda: Xarxa ja existent (cable propi) Xarxa ja existent (disponibilitat de fibres a cable de tercers) Xarxa desplegada per la SPD el 2020 Xarxa desplegada per la XOC el 2020 Calendari de recepció d’obres: • El Vendrell – Valls: 31/12/2020. • Valls - Montblanc: 31/12/2020. • Tortosa – Gandesa: 31/12/2020. • Mora la Nova – Falset: 31/01/2021. A disposició del mercat majorista gener/2021 (22/gener) 3 Desplegament 2020 (II). Instruments de comercialització. Llegenda: Xarxa ja existent (cable propi) Xarxa ja existent (disponibilitat de fibres a cable de tercers) Xarxa desplegada per la SPD el 2020 Xarxa desplegada per la XOC el 2020 Instruments de comercialització: Xarxa desplegada per la SPD el 2020 • Preu públic CTTI de lloguer de conductes: 0,53 €/m/any amb bonificacions de fins el 50% en funció de la densitat i número d’habitants del terme municipal. • Nou preu públic CTTI de lloguer de fibres fosques (finals gener) Sol·licituds via el Punt d’Informació Únic (PIU) • https://politiquesdigitals.gencat.cat/ca/tic/piu/ Xarxa desplegada per la XOC el 2020 • Oferta majorista de lloguer de fibres fosques • Oferta majorista de serveis actius • https://www.xarxaoberta.cat/ 4 Desplegament 2020 (II) Els -

Bibliografia Histórica Tarraconense Xii
INSTITUT PE ru r TARR~ ONENSES RAM N JBJERENGUER .IV BIBLIOGRAFIA HISTÓRICA TARRACONENSE XII E DICIÓ A CURA DE F. X AVIER RICOMÁ Y END RELL 1 S ALVADOR-J . R üVIRA 1 ÜÓMEZ EXCMA. DIPUTACIÓ DE TARRAGONA 1990 BIBLIOGRAFIA HISTÓRICA TARRACONENSE XII INSTITUT D 'ESTUDIS TARRACONENSES RAMON BERENGUER IV SECCIÓ DE BIOGRAFIA I BIBLIOGRAFIA PUBLICACIÓ NÚM. 17 BIBLIOGRAFIA HISTÓRICA TARRACONENSE XII EDICJÓ A CURA DE F. X A VIER RICOMÁ Y ENDRELL l SALVADOR-J . R OVI RA l G ó MEZ EXCMA. DIPUTACIÓ DE TARRAGONA 1990 Institut d'Estudis Tarraconenses Ramon Berenguer IV Publicació núm. 170 ISSN: 0211 - 1438 Diposit Legal: T. 1.527. 1990 Ho edita: lnstitut d'Estudis Tarraconenses Ramon Berenguer IV. Santa Anna, 8 Tel. 23 50 32 - 43003 Tarragona Imprés a Indústries Gráfiques Gabriel Gibert, S. A. - Cartagena, 12 - Tarragona ÍNDEX Col·laboradors d'aquest número 9 Publicacions ressenyades 11 Arqueología 15 Art 17 Biografíes 29 Ciencies auxiliars 37 Cultura 47 Demografía 49 Economía i societat 53 Etnografía 65 Fonts i bibliografía 71 Historia eclesiastica 73 Historia local 83 Historia política i militar 135 Índex d'autors ressenyats 139 Índex de !loes 143 Índex de persones 147 La Bibliografía Histórica Tarraconense és un repertori bibliografic recollit i editat per la Secció de Biografía i Bibliografía de l'Institut d' Es tudis Tarraconenses Ramon Berenguer IV. Aplega ressenyes de treballs histories apareguts durant l' any abans a la data de publicació. No obstant aixo, també hi són aplegades les deis publicats amb anterioritat i que, o bé no foren coneguts per la redacció o bé se li feren a mans quan l'edició ja era closa. -

Centres-Tarragona.Pdf
Relació de centres formadors autoritzats pel Departament d’Educació 2019-2020 Serveis Territorials a Tarragona Codi del centre Nom del centre Població 43006174 Escola Les Moreres Aiguamúrcia 43000020 Escola Sant Miquel Aiguamúrcia 43005297 Escola Joan Perucho Albinyana 43010633 Llar d'infants d'Albinyana Albinyana 43000135 Escola Mare de Déu del Remei Alcover 43009497 Institut Fonts del Glorieta Alcover 43007464 Llar d'infants Xiu-xiu Alcover 43000214 Escola Josep Fusté Alforja 43005327 Escola Ramon Sugrañes Almoster 43000251 Escola La Portalada Altafulla 43011297 Llar d'infants Hort de Pau Altafulla 43005133 Escola Mare de Déu del Priorat Banyeres del Penedès 43009709 Llar d'infants de Banyeres del Penedès Banyeres del Penedès 43000548 Escola La Muntanyeta Bellvei 43007506 Llar d'infants Municipal Bellvei 43000640 Escola Mare de Déu de la Candela Botarell 43007440 Llar d'infants Els Patufets Botarell 43000676 Escola El Castell - ZER Montsant Cabacés 43011303 ZER Montsant Cabacés 43011285 Llar d'infants Les Cabretes Cabra del Camp 43009898 Escola Castell de Calafell Calafell 43010098 Escola La Ginesta Calafell 43000721 Escola Mossèn Jacint Verdaguer Calafell 43000706 Escola Santa Creu de Calafell Calafell 43011121 Escola Vilamar Calafell 43007257 Institut Camí de Mar Calafell 43010372 Institut La Talaia Calafell 43000755 Cardenal Vidal i Barraquer Cambrils 43008547 Escola Cambrils Cambrils 43010141 Escola Guillem Fortuny Cambrils 43000731 Escola Joan Ardèvol Cambrils 43011212 Escola La Bòbila Cambrils 43006356 Escola Marinada Cambrils 43010581 Escola Mas Clariana Cambrils 43006654 Institut Cambrils Cambrils 43007038 Institut Escola d'Hoteleria i Turisme Cambrils 43010335 Institut La Mar de la Frau Cambrils 43000779 Institut Ramon Berenguer IV Cambrils 43012149 Llar d'infants La Galereta Cambrils 43011078 Llar d'infants M. -

Rankings Province of Tarragona
10/9/2021 Maps, analysis and statistics about the resident population Demographic balance, population and familiy trends, age classes and average age, civil status and foreigners Skip Navigation Links SPAGNA / CATALUÑA / Province of TARRAGONA Powered by Page 1 L'azienda Contatti Login Urbistat on Linkedin Adminstat logo DEMOGRAPHY ECONOMY RANKINGS SEARCH SPAGNA Municipalities Powered by Page 2 AIGUAMÚRCIA Stroll up beside >> L'azienda Contatti Login Urbistat on Linkedin FLIX AdminstatALBINYANA logo DEMOGRAPHY ECONOMY RANKINGS SEARCH FORÈS ALCANAR SPAGNA FREGINALS ALCOVER GANDESA ALDOVER GARCIA ALFARA DE CARLES GINESTAR ALFORJA GODALL ALIÓ GRATALLOPS ALMOSTER HORTA DE SANT JOAN ALTAFULLA L'ALBIOL AMPOSTA L'ALDEA ARBOLÍ L'ALEIXAR ARNES L'AMETLLA DE ASCÓ MAR BANYERES L'AMPOLLA DEL PENEDÈS L'ARBOÇ BARBERÀ DE LA CONCA L'ARGENTERA BATEA L'ESPLUGA DE FRANCOLÍ BELLMUNT DEL PRIORAT LA BISBAL DE FALSET BELLVEI LA BISBAL DEL BENIFALLET PENEDÈS BENISSANET LA CANONJA BLANCAFORT LA FATARELLA BONASTRE LA FEBRÓ BOT LA FIGUERA BOTARELL LA GALERA BRÀFIM LA MASÓ CABACÉS LA MORERA DE CABRA DEL MONTSANT CAMP LA NOU DE CALAFELL GAIÀ CAMARLES LA PALMA CAMBRILS D'EBRE CAPAFONTS LA POBLA DE CAPÇANES MAFUMET Powered by Page 3 CASERES LA POBLA DE L'azienda Contatti Login Urbistat on Linkedin MASSALUCA CASTELLVELL Adminstat logo DEL CAMP LA POBLA DEDEMOGRAPHY ECONOMY RANKINGS SEARCH SPAGNA MONTORNÈS COLLDEJOU LA RIBA CONESA LA RIERA DE CONSTANTÍ GAIÀ CORBERA LA SECUITA D'EBRE LA SELVA DEL CORNUDELLA CAMP DE MONTSANT LA SÉNIA CREIXELL LA TORRE DE CUNIT FONTAUBELLA