Chapter 2 Approximate Thermodynamics
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Potential Vorticity
POTENTIAL VORTICITY Roger K. Smith March 3, 2003 Contents 1 Potential Vorticity Thinking - How might it help the fore- caster? 2 1.1Introduction............................ 2 1.2WhatisPV-thinking?...................... 4 1.3Examplesof‘PV-thinking’.................... 7 1.3.1 A thought-experiment for understanding tropical cy- clonemotion........................ 7 1.3.2 Kelvin-Helmholtz shear instability . ......... 9 1.3.3 Rossby wave propagation in a β-planechannel..... 12 1.4ThestructureofEPVintheatmosphere............ 13 1.4.1 Isentropicpotentialvorticitymaps........... 14 1.4.2 The vertical structure of upper-air PV anomalies . 18 2 A Potential Vorticity view of cyclogenesis 21 2.1PreliminaryIdeas......................... 21 2.2SurfacelayersofPV....................... 21 2.3Potentialvorticitygradientwaves................ 23 2.4 Baroclinic Instability . .................... 28 2.5 Applications to understanding cyclogenesis . ......... 30 3 Invertibility, iso-PV charts, diabatic and frictional effects. 33 3.1 Invertibility of EPV ........................ 33 3.2Iso-PVcharts........................... 33 3.3Diabaticandfrictionaleffects.................. 34 3.4Theeffectsofdiabaticheatingoncyclogenesis......... 36 3.5Thedemiseofcutofflowsandblockinganticyclones...... 36 3.6AdvantageofPVanalysisofcutofflows............. 37 3.7ThePVstructureoftropicalcyclones.............. 37 1 Chapter 1 Potential Vorticity Thinking - How might it help the forecaster? 1.1 Introduction A review paper on the applications of Potential Vorticity (PV-) concepts by Brian -
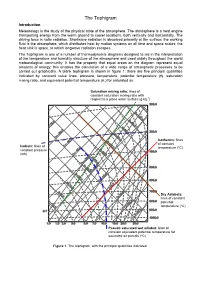
The Tephigram Introduction Meteorology Is the Study of the Physical State of the Atmosphere
The Tephigram Introduction Meteorology is the study of the physical state of the atmosphere. The atmosphere is a heat engine transporting energy from the warm ground to cooler locations, both vertically and horizontally. The driving force is solar radiation. Shortwave radiation is absorbed primarily at the surface; the working fluid is the atmosphere, which distributes heat by motion systems on all time and space scales; the heat sink is space, to which longwave radiation escapes. The Tephigram is one of a number of thermodynamic diagrams designed to aid in the interpretation of the temperature and humidity structure of the atmosphere and used widely throughout the world meteorological community. It has the property that equal areas on the diagram represent equal amounts of energy; this enables the calculation of a wide range of atmospheric processes to be carried out graphically. A blank tephigram is shown in figure 1; there are five principal quantities indicated by constant value lines: pressure, temperature, potential temperature (θ), saturation mixing ratio, and equivalent potential temperature (θe) for saturated air. Saturation mixing ratio: lines of constant saturation mixing ratio with respect to a plane water surface (g kg-1) Isotherms: lines of constant Isobars: lines of temperature (ºC) constant pressure (mb) Dry Adiabats: lines of constant potential temperature (ºC) Pseudo saturated wet adiabat: lines of constant equivalent potential temperature for saturated air parcels (ºC) Figure 1. The tephigram, with the principal quantities indicated. The principal axes of a tephigram are temperature and potential temperature; these are straight and perpendicular to each other, but rotated through about 45º anticlockwise so that lines of constant temperature run from bottom left to top right on the diagram. -

Gibbs Seawater (GSW) Oceanographic Toolbox of TEOS–10
Gibbs SeaWater (GSW) Oceanographic Toolbox of TEOS–10 documentation set density and enthalpy, based on the 48-term expression for density, gsw_front_page front page to the GSW Oceanographic Toolbox The functions in this group ending in “_CT” may also be called without “_CT”. gsw_contents contents of the GSW Oceanographic Toolbox gsw_check_functions checks that all the GSW functions work correctly gsw_rho_CT in-situ density, and potential density gsw_demo demonstrates many GSW functions and features gsw_alpha_CT thermal expansion coefficient with respect to CT gsw_beta_CT saline contraction coefficient at constant CT Practical Salinity (SP), PSS-78 gsw_rho_alpha_beta_CT in-situ density, thermal expansion & saline contraction coefficients gsw_specvol_CT specific volume gsw_SP_from_C Practical Salinity from conductivity, C (incl. for SP < 2) gsw_specvol_anom_CT specific volume anomaly gsw_C_from_SP conductivity, C, from Practical Salinity (incl. for SP < 2) gsw_sigma0_CT sigma0 from CT with reference pressure of 0 dbar gsw_SP_from_R Practical Salinity from conductivity ratio, R (incl. for SP < 2) gsw_sigma1_CT sigma1 from CT with reference pressure of 1000 dbar gsw_R_from_SP conductivity ratio, R, from Practical Salinity (incl. for SP < 2) gsw_sigma2_CT sigma2 from CT with reference pressure of 2000 dbar gsw_SP_salinometer Practical Salinity from a laboratory salinometer (incl. for SP < 2) gsw_sigma3_CT sigma3 from CT with reference pressure of 3000 dbar gsw_sigma4_CT sigma4 from CT with reference pressure of 4000 dbar Absolute Salinity -

Dry Adiabatic Temperature Lapse Rate
ATMO 551a Fall 2010 Dry Adiabatic Temperature Lapse Rate As we discussed earlier in this class, a key feature of thick atmospheres (where thick means atmospheres with pressures greater than 100-200 mb) is temperature decreases with increasing altitude at higher pressures defining the troposphere of these planets. We want to understand why tropospheric temperatures systematically decrease with altitude and what the rate of decrease is. The first order explanation is the dry adiabatic lapse rate. An adiabatic process means no heat is exchanged in the process. For this to be the case, the process must be “fast” so that no heat is exchanged with the environment. So in the first law of thermodynamics, we can anticipate that we will set the dQ term equal to zero. To get at the rate at which temperature decreases with altitude in the troposphere, we need to introduce some atmospheric relation that defines a dependence on altitude. This relation is the hydrostatic relation we have discussed previously. In summary, the adiabatic lapse rate will emerge from combining the hydrostatic relation and the first law of thermodynamics with the heat transfer term, dQ, set to zero. The gravity side The hydrostatic relation is dP = −g ρ dz (1) which relates pressure to altitude. We rearrange this as dP −g dz = = α dP (2) € ρ where α = 1/ρ is known as the specific volume which is the volume per unit mass. This is the equation we will use in a moment in deriving the adiabatic lapse rate. Notice that € F dW dE g dz ≡ dΦ = g dz = g = g (3) m m m where Φ is known as the geopotential, Fg is the force of gravity and dWg is the work done by gravity. -

Introduction to Atmospheric Dynamics Chapter 2
Introduction to Atmospheric Dynamics Chapter 2 Paul A. Ullrich [email protected] Part 3: Buoyancy and Convection Vertical Structure This cooling with height is related to the dynamics of the atmosphere. The change of temperature with height is called the lapse rate. Defnition: The lapse rate is defned as the rate (for instance in K/km) at which temperature decreases with height. @T Γ ⌘@z Paul Ullrich Introduction to Atmospheric Dynamics March 2014 Lapse Rate For a dry adiabatic, stable, hydrostatic atmosphere the potential temperature θ does not vary in the vertical direction: @✓ =0 @z In a dry adiabatic, hydrostatic atmosphere the temperature T must decrease with height. How quickly does the temperature decrease? R/cp p0 Note: Use ✓ = T p ✓ ◆ Paul Ullrich Introduction to Atmospheric Dynamics March 2014 Lapse Rate The adiabac change in temperature with height is T @✓ @T g = + ✓ @z @z cp For dry adiabac, hydrostac atmosphere: @T g = Γd − @z cp ⌘ Defnition: The dry adiabatic lapse rate is defned as the g rate (for instance in K/km) at which the temperature of an Γd air parcel will decrease with height if raised adiabatically. ⌘ cp g 1 9.8Kkm− cp ⇡ Paul Ullrich Introduction to Atmospheric Dynamics March 2014 Lapse Rate This profle should be very close to the adiabatic lapse rate in a dry atmosphere. Paul Ullrich Introduction to Atmospheric Dynamics March 2014 Fundamentals Even in adiabatic motion, with no external source of heating, if a parcel moves up or down its temperature will change. • What if a parcel moves about a surface of constant pressure? • What if a parcel moves about a surface of constant height? If the atmosphere is in adiabatic balance, the temperature still changes with height. -

Spiciness Theory Revisited, with New Views on Neutral Density, Orthogonality, and Passiveness
Ocean Sci., 17, 203–219, 2021 https://doi.org/10.5194/os-17-203-2021 © Author(s) 2021. This work is distributed under the Creative Commons Attribution 4.0 License. Spiciness theory revisited, with new views on neutral density, orthogonality, and passiveness Rémi Tailleux Dept. of Meteorology, University of Reading, Earley Gate, RG6 6ET Reading, United Kingdom Correspondence: Rémi Tailleux ([email protected]) Received: 26 April 2020 – Discussion started: 20 May 2020 Revised: 8 December 2020 – Accepted: 9 December 2020 – Published: 28 January 2021 Abstract. This paper clarifies the theoretical basis for con- 1 Introduction structing spiciness variables optimal for characterising ocean water masses. Three essential ingredients are identified: (1) a As is well known, three independent variables are needed to material density variable γ that is as neutral as feasible, (2) a fully characterise the thermodynamic state of a fluid parcel in material state function ξ independent of γ but otherwise ar- the standard approximation of seawater as a binary fluid. The bitrary, and (3) an empirically determined reference function standard description usually relies on the use of a tempera- ξr(γ / of γ representing the imagined behaviour of ξ in a ture variable (such as potential temperature θ, in situ temper- notional spiceless ocean. Ingredient (1) is required because ature T , or Conservative Temperature 2), a salinity variable contrary to what is often assumed, it is not the properties im- (such as reference composition salinity S or Absolute Salin- posed on ξ (such as orthogonality) that determine its dynam- ity SA), and pressure p. -

Equations of Motion Using Thermodynamic Coordinates
2814 JOURNAL OF PHYSICAL OCEANOGRAPHY VOLUME 30 Equations of Motion Using Thermodynamic Coordinates ROLAND A. DE SZOEKE College of Oceanic and Atmospheric Sciences, Oregon State University, Corvallis, Oregon (Manuscript received 21 October 1998, in ®nal form 30 December 1999) ABSTRACT The forms of the primitive equations of motion and continuity are obtained when an arbitrary thermodynamic state variableÐrestricted only to be vertically monotonicÐis used as the vertical coordinate. Natural general- izations of the Montgomery and Exner functions suggest themselves. For a multicomponent ¯uid like seawater the dependence of the coordinate on salinity, coupled with the thermobaric effect, generates contributions to the momentum balance from the salinity gradient, multiplied by a thermodynamic coef®cient that can be com- pletely described given the coordinate variable and the equation of state. In the vorticity balance this term produces a contribution identi®ed with the baroclinicity vector. Only when the coordinate variable is a function only of pressure and in situ speci®c volume does the coef®cient of salinity gradient vanish and the baroclinicity vector disappear. This coef®cient is explicitly calculated and displayed for potential speci®c volume as thermodynamic coor- dinate, and for patched potential speci®c volume, where different reference pressures are used in various pressure subranges. Except within a few hundred decibars of the reference pressures, the salinity-gradient coef®cient is not negligible and ought to be taken into account in ocean circulation models. 1. Introduction perature surfaces is a potential for the acceleration ®eld, Potential temperature, the temperature a ¯uid parcel when friction is negligible. Because the gradient of this would have if removed adiabatically and reversibly from function gives the geostrophic ¯ow when it balances ambient pressure to a reference pressure, is a valuable Coriolis force, it is also called the geostrophic stream- concept in the study of the atmosphere and oceans. -

Recovery of Temperature, Salinity, and Potential Density from Ocean
PUBLICATIONS Journal of Geophysical Research: Oceans RESEARCH ARTICLE Recovery of temperature, salinity, and potential density from 10.1002/2013JC009662 ocean reflectivity Key Points: Berta Biescas1,2, Barry R. Ruddick1, Mladen R. Nedimovic3,4,5, Valentı Sallare`s2, Guillermo Bornstein2, Recovery of oceanic T, S, and and Jhon F. Mojica2 potential density from acoustic reflectivity data 1Department of Oceanography, Dalhousie University, Halifax, Nova Scotia, Canada, 2Department of Marine Geology, Thermal anomalies observations at 3 the Mediterranean tongue level Institut de Cie`ncies del Mar ICM-CSIC, Barcelona, Spain, Department of Earth’s Sciences, Dalhousie University, Halifax, 4 5 Comparison between acoustic Nova Scotia, Canada, Lamont-Doherty Earth Observatory of Columbia University, Palisades, New York, USA, University of reflectors in the ocean and isopycnals Texas Institute for Geophysics, Austin, Texas, USA Supporting Information: Methods Abstract This work explores a method to recover temperature, salinity, and potential density of the Supporting figures ocean using acoustic reflectivity data and time and space coincident expendable bathythermographs (XBT). The acoustically derived (vertical frequency >10 Hz) and the XBT-derived (vertical frequency <10 Hz) impe- Correspondence to: dances are summed in the time domain to form impedance profiles. Temperature (T) and salinity (S) are B. Biescas, then calculated from impedance using the international thermodynamics equations of seawater (GSW [email protected] TEOS-10) and an empirical T-S relation derived with neural networks; and finally potential density (q) is cal- culated from T and S. The main difference between this method and previous inversion works done from Citation: Biescas, B., B. R. Ruddick, M. R. real multichannel seismic reflection (MCS) data recorded in the ocean, is that it inverts density and it does Nedimovic, V. -

Eddy Activity Sensitivity to Changes in the Vertical Structure of Baroclinicity
APRIL 2016 Y U V A L A N D K A S P I 1709 Eddy Activity Sensitivity to Changes in the Vertical Structure of Baroclinicity JANNI YUVAL AND YOHAI KASPI Department of Earth and Planetary Sciences, Weizmann Institute of Science, Rehovot, Israel (Manuscript received 12 May 2015, in final form 25 October 2015) ABSTRACT The relation between the mean meridional temperature gradient and eddy fluxes has been addressed by several eddy flux closure theories. However, these theories give little information on the dependence of eddy fluxes on the vertical structure of the temperature gradient. The response of eddies to changes in the vertical structure of the temperature gradient is especially interesting since global circulation models suggest that as a result of greenhouse warming, the lower-tropospheric temperature gradient will decrease whereas the upper- tropospheric temperature gradient will increase. The effects of the vertical structure of baroclinicity on at- mospheric circulation, particularly on the eddy activity, are investigated. An idealized global circulation model with a modified Newtonian relaxation scheme is used. The scheme allows the authors to obtain a heating profile that produces a predetermined mean temperature profile and to study the response of eddy activity to changes in the vertical structure of baroclinicity. The results indicate that eddy activity is more sensitive to temperature gradient changes in the upper troposphere. It is suggested that the larger eddy sensitivity to the upper-tropospheric temperature gradient is a consequence of large baroclinicity concen- trated in upper levels. This result is consistent with a 1D Eady-like model with nonuniform shear showing more sensitivity to shear changes in regions of larger baroclinicity. -

Physical Properties of Seawater
CHAPTER 3 Physical Properties of Seawater 3.1. MOLECULAR PROPERTIES in the oceans, because water is such an effective OF WATER heat reservoir (see Section S15.6 located on the textbook Web site Many of the unique characteristics of the ; “S” denotes supplemental ocean can be ascribed to the nature of water material). itself. Consisting of two positively charged As seawater is heated, molecular activity hydrogen ions and a single negatively charged increases and thermal expansion occurs, oxygen ion, water is arranged as a polar mole- reducing the density. In freshwater, as tempera- cule having positive and negative sides. This ture increases from the freezing point up to about molecular polarity leads to water’s high dielec- 4C, the added heat energy forms molecular tric constant (ability to withstand or balance an chains whose alignment causes the water to electric field). Water is able to dissolve many shrink, increasing the density. As temperature substances because the polar water molecules increases above this point, the chains break align to shield each ion, resisting the recombina- down and thermal expansion takes over; this tion of the ions. The ocean’s salty character is explains why fresh water has a density maximum due to the abundance of dissolved ions. at about 4C rather than at its freezing point. In The polar nature of the water molecule seawater, these molecular effects are combined causes it to form polymer-like chains of up to with the influence of salt, which inhibits the eight molecules. Approximately 90% of the formation of the chains. For the normal range of water molecules are found in these chains. -

Nomaly Patterns in the Near-Surface Baroclinicity And
1 Dominant Anomaly Patterns in the Near-Surface Baroclinicity and 2 Accompanying Anomalies in the Atmosphere and Oceans. Part II: North 3 Pacific Basin ∗ † 4 Mototaka Nakamura and Shozo Yamane 5 Japan Agency for Marine-Earth Science and Technology, Yokohama, Kanagawa, Japan ∗ 6 Corresponding author address: Mototaka Nakamura, Japan Agency for Marine-Earth Science and 7 Technology, 3173-25 Showa-machi, Kanazawa-ku, Yokohama, Kanagawa 236-0001, Japan. 8 E-mail: [email protected] † 9 Current affiliation: Science and Engineering, Doshisha University, Kyotanabe, Kyoto, Japan. Generated using v4.3.2 of the AMS LATEX template 1 ABSTRACT 10 Variability in the monthly-mean flow and storm track in the North Pacific 11 basin is examined with a focus on the near-surface baroclinicity. Dominant 12 patterns of anomalous near-surface baroclinicity found from EOF analyses 13 generally show mixed patterns of shift and changes in the strength of near- 14 surface baroclinicity. Composited anomalies in the monthly-mean wind at 15 various pressure levels based on the signals in the EOFs show accompany- 16 ing anomalies in the mean flow up to 50 hPa in the winter and up to 100 17 hPa in other seasons. Anomalous eddy fields accompanying the anomalous 18 near-surface baroclinicity patterns exhibit, broadly speaking, structures antic- 19 ipated from simple linear theories of baroclinic instability, and suggest a ten- 20 dency for anomalous wave fluxes to accelerate–decelerate the surface west- 21 erly accordingly. However, the relationship between anomalous eddy fields 22 and anomalous near-surface baroclinicity in the midwinter is not consistent 23 with the simple linear baroclinic instability theories. -

Is the Neutral Surface Really Neutral? a Close Examination of Energetics of Along Isopycnal Mixing
Is the neutral surface really neutral? A close examination of energetics of along isopycnal mixing Rui Xin Huang Department of Physical Oceanography Woods Hole Oceanographic Institution Woods Hole, MA 02543, USA April 19, 2010 Abstract The concept of along-isopycnal (along-neutral-surface) mixing has been one of the foundations of the classical large-scale oceanic circulation framework. Neutral surface has been defined a surface that a water parcel move a small distance along such a surface does not require work against buoyancy. A close examination reveals two important issues. First, due to mass continuity it is meaningless to discuss the consequence of moving a single water parcel. Instead, we have to deal with at least two parcels in discussing the movement of water masses and energy change of the system. Second, movement along the so-called neutral surface or isopycnal surface in most cases is associated with changes in the total gravitational potential energy of the ocean. In light of this discovery, neutral surface may be treated as a preferred surface for the lateral mixing in the ocean. However, there is no neutral surface even at the infinitesimal sense. Thus, although approximate neutral surfaces can be defined for the global oceans and used in analysis of global thermohaline circulation, they are not the surface along which water parcels actually travel. In this sense, thus, any approximate neutral surface can be used, and their function is virtually the same. Since the structure of the global thermohaline circulation change with time, the most suitable option of quasi-neutral surface is the one which is most flexible and easy to be implemented in numerical models.