References: Humidity Variables
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Thermodynamics of Solar Energy Conversion in to Work
Sri Lanka Journal of Physics, Vol. 9 (2008) 47-60 Institute of Physics - Sri Lanka Research Article Thermodynamic investigation of solar energy conversion into work W.T.T. Dayanga and K.A.I.L.W. Gamalath Department of Physics, University of Colombo, Colombo 03, Sri Lanka Abstract Using a simple thermodynamic upper bound efficiency model for the conversion of solar energy into work, the best material for a converter was obtained. Modifying the existing detailed terrestrial application model of direct solar radiation to include an atmospheric transmission coefficient with cloud factors and a maximum concentration ratio, the best shape for a solar concentrator was derived. Using a Carnot engine in detailed space application model, the best shape for the mirror of a concentrator was obtained. A new conversion model was introduced for a solar chimney power plant to obtain the efficiency of the power plant and power output. 1. INTRODUCTION A system that collects and converts solar energy in to mechanical or electrical power is important from various aspects. There are two major types of solar power systems at present, one using photovoltaic cells for direct conversion of solar radiation energy in to electrical energy in combination with electrochemical storage and the other based on thermodynamic cycles. The efficiency of a solar thermal power plant is significantly higher [1] compared to the maximum efficiency of twenty percent of a solar cell. Although the initial cost of a solar thermal power plant is very high, the running cost is lower compared to the other power plants. Therefore most countries tend to build solar thermal power plants. -

Solar Thermal Energy
22 Solar Thermal Energy Solar thermal energy is an application of solar energy that is very different from photovol- taics. In contrast to photovoltaics, where we used electrodynamics and solid state physics for explaining the underlying principles, solar thermal energy is mainly based on the laws of thermodynamics. In this chapter we give a brief introduction to that field. After intro- ducing some basics in Section 22.1, we will discuss Solar Thermal Heating in Section 22.2 and Concentrated Solar (electric) Power (CSP) in Section 22.3. 22.1 Solar thermal basics We start this section with the definition of heat, which sometimes also is called thermal energy . The molecules of a body with a temperature different from 0 K exhibit a disordered movement. The kinetic energy of this movement is called heat. The average of this kinetic energy is related linearly to the temperature of the body. 1 Usually, we denote heat with the symbol Q. As it is a form of energy, its unit is Joule (J). If two bodies with different temperatures are brought together, heat will flow from the hotter to the cooler body and as a result the cooler body will be heated. Dependent on its physical properties and temperature, this heat can be absorbed in the cooler body in two forms, sensible heat and latent heat. Sensible heat is that form of heat that results in changes in temperature. It is given as − Q = mC p(T2 T1), (22.1) where Q is the amount of heat that is absorbed by the body, m is its mass, Cp is its heat − capacity and (T2 T1) is the temperature difference. -

Investigating Applicability of Evaporative Cooling Systems for Thermal Comfort of Poultry Birds in Pakistan
applied sciences Article Investigating Applicability of Evaporative Cooling Systems for Thermal Comfort of Poultry Birds in Pakistan Hafiz M. U. Raza 1, Hadeed Ashraf 1, Khawar Shahzad 1, Muhammad Sultan 1,* , Takahiko Miyazaki 2,3, Muhammad Usman 4,* , Redmond R. Shamshiri 5 , Yuguang Zhou 6 and Riaz Ahmad 6 1 Department of Agricultural Engineering, Bahauddin Zakariya University, Bosan Road, Multan 60800, Pakistan; [email protected] (H.M.U.R.); [email protected] (H.A.); [email protected] (K.S.) 2 Faculty of Engineering Sciences, Kyushu University, Kasuga-koen 6-1, Kasuga-shi, Fukuoka 816-8580, Japan; [email protected] 3 International Institute for Carbon-Neutral Energy Research (WPI-I2CNER), Kyushu University, 744 Motooka, Nishi-ku, Fukuoka 819-0395, Japan 4 Institute for Water Resources and Water Supply, Hamburg University of Technology, Am Schwarzenberg-Campus 3, 20173 Hamburg, Germany 5 Leibniz Institute for Agricultural Engineering and Bioeconomy, Max-Eyth-Allee 100, 14469 Potsdam-Bornim, Germany; [email protected] 6 Bioenergy and Environment Science & Technology Laboratory, College of Engineering, China Agricultural University, Beijing 100083, China; [email protected] (Y.Z.); [email protected] (R.A.) * Correspondence: [email protected] (M.S.); [email protected] (M.U.); Tel.: +92-333-610-8888 (M.S.); Fax: +92-61-9210298 (M.S.) Received: 4 June 2020; Accepted: 24 June 2020; Published: 28 June 2020 Abstract: In the 21st century, the poultry sector is a vital concern for the developing economies including Pakistan. The summer conditions of the city of Multan (Pakistan) are not comfortable for poultry birds. -
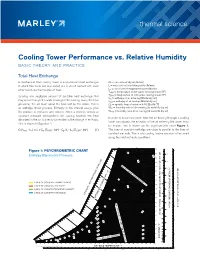
Thermal Science Cooling Tower Performance Vs. Relative Humidity
thermal science Cooling Tower Performance vs. Relative Humidity BASIC THEORY AND PRACTICE Total Heat Exchange A mechanical draft cooling tower is a specialized heat exchanger G = mass rate of dry air [lb/min] in which two fluids (air and water) are in direct contact with each L = mass rate of circulating water [lb/min] other to induce the transfer of heat. Le = mass rate of evaporated water [lb/min] THW = temperature of hot water entering tower [°F] Ignoring any negligible amount of sensible heat exchange that TCW = temperature of cold water leaving tower [°F] hin = enthalpy of air entering [Btu/lb/dry air] may occur through the walls (casing) of the cooling tower, the heat hout = enthalpy of air leaving [Btu/lb/dry air] gained by the air must equal the heat lost by the water. This is Cp = specific heat of water = 4.18 [Btu/lb-°F] an enthalpy driven process. Enthalpy is the internal energy plus Win = humidity ratio of air entering [lb water/lb dry air] the product of pressure and volume. When a process occurs at Wout = humidity ratio of air leaving [lb water/lb dry air] constant pressure (atmospheric for cooling towers), the heat In order to know how much heat the air flowing through a cooling absorbed in the air is directly correlated to the change in enthalpy. tower can absorb, the enthalpy of the air entering the tower must This is shown in Equation 1. be known. This is shown on the psychrometric chart Figure 1. G (hout - hin) = L x Cp (THW - 32°) - Cp (L - Le)(TCW - 32°) (1) The lines of constant enthalpy are close to parallel to the lines of constant wet bulb. -

Recommended Qualification Test Procedure for Solar Absorber.Pdf
Date: 2004-09-10 Editor Bo Carlsson1 IEA Solar Heating and Cooling Program Task 27 Performance of Solar Façade Components Project: Service life prediction tools for Solar Collectors Recommended qualification test procedure for solar absorber surface durability 1 Address: SP Swedish National Testing and Research Institute, P.O.Box 857, SE-50115 Borås, e-mail: [email protected] Contents Page Foreword.............................................................................................................................................................iv Introduktion .........................................................................................................................................................v 1 Scope ......................................................................................................................................................1 2 Normative references ............................................................................................................................1 3 Terms and definitions ...........................................................................................................................2 4 Requirements and classification..........................................................................................................3 5 Test methods for assessing material properties as measure of absorber performance...............4 5.1 Sampling and preparation of test specimens.....................................................................................4 -

Chapter 8 Atmospheric Statics and Stability
Chapter 8 Atmospheric Statics and Stability 1. The Hydrostatic Equation • HydroSTATIC – dw/dt = 0! • Represents the balance between the upward directed pressure gradient force and downward directed gravity. ρ = const within this slab dp A=1 dz Force balance p-dp ρ p g d z upward pressure gradient force = downward force by gravity • p=F/A. A=1 m2, so upward force on bottom of slab is p, downward force on top is p-dp, so net upward force is dp. • Weight due to gravity is F=mg=ρgdz • Force balance: dp/dz = -ρg 2. Geopotential • Like potential energy. It is the work done on a parcel of air (per unit mass, to raise that parcel from the ground to a height z. • dφ ≡ gdz, so • Geopotential height – used as vertical coordinate often in synoptic meteorology. ≡ φ( 2 • Z z)/go (where go is 9.81 m/s ). • Note: Since gravity decreases with height (only slightly in troposphere), geopotential height Z will be a little less than actual height z. 3. The Hypsometric Equation and Thickness • Combining the equation for geopotential height with the ρ hydrostatic equation and the equation of state p = Rd Tv, • Integrating and assuming a mean virtual temp (so it can be a constant and pulled outside the integral), we get the hypsometric equation: • For a given mean virtual temperature, this equation allows for calculation of the thickness of the layer between 2 given pressure levels. • For two given pressure levels, the thickness is lower when the virtual temperature is lower, (ie., denser air). • Since thickness is readily calculated from radiosonde measurements, it provides an excellent forecasting tool. -

Evaluation of the Effect of Relative Humidity of Air on the Coefficients of Critical Flow Venturi Nozzles
Evaluation of the Effect of Relative Humidity of Air on the Coefficients of Critical Flow Venturi Nozzles K. Chahine and M. Ballico National Measurement Institute, Australia P O Box 264, Lindfield, NSW 2070, Australia [email protected] Abstract atmospheric pressures. To establish stable sonic conditions in the nozzle, the down-stream end of the nozzle is usually connected to a high-capacity vacuum At NMIA, volumetric standards such as Brooks pump, with the up-stream end connected to the meter- or bell provers are used to calibrate critical flow Venturi under-test. During calibration the test-flowmeter draws nozzles or “sonic nozzles”. These nozzles, which are air from the laboratory at or near atmospheric pressures extremely stable, are used by both NMIA and Australian and temperature, and with relative humidity varying accredited laboratories to establish continuous flows for between 40% and 60%. The mass flowrate produced by the calibration of gas flow meters. For operational the nozzles is calculated based on the calibrated values of reasons, sonic nozzles are generally calibrated using dry the nozzle coefficient, the measured up-stream pressure air but later used with standard atmospheric air at various and the density calculated from the air temperature, humidity levels either drawn or blown through the meter- pressure and humidity [2]. under-test. Although the accepted theoretical calculations for determining the mass flow through a sonic nozzle At present, any effect of the relative humidity on the incorporate corrections for the resulting change in air nozzle coefficients is considered as negligible, as various density, as laboratories seek to reduce uncertainties the authors have estimated the systematic error at 0.02% for validity of this assumption warrants further examination. -

Comparison Between Observed Convective Cloud-Base Heights and Lifting Condensation Level for Two Different Lifted Parcels
AUGUST 2002 NOTES AND CORRESPONDENCE 885 Comparison between Observed Convective Cloud-Base Heights and Lifting Condensation Level for Two Different Lifted Parcels JEFFREY P. C RAVEN AND RYAN E. JEWELL NOAA/NWS/Storm Prediction Center, Norman, Oklahoma HAROLD E. BROOKS NOAA/National Severe Storms Laboratory, Norman, Oklahoma 6 January 2002 and 16 April 2002 ABSTRACT Approximately 400 Automated Surface Observing System (ASOS) observations of convective cloud-base heights at 2300 UTC were collected from April through August of 2001. These observations were compared with lifting condensation level (LCL) heights above ground level determined by 0000 UTC rawinsonde soundings from collocated upper-air sites. The LCL heights were calculated using both surface-based parcels (SBLCL) and mean-layer parcels (MLLCLÐusing mean temperature and dewpoint in lowest 100 hPa). The results show that the mean error for the MLLCL heights was substantially less than for SBLCL heights, with SBLCL heights consistently lower than observed cloud bases. These ®ndings suggest that the mean-layer parcel is likely more representative of the actual parcel associated with convective cloud development, which has implications for calculations of thermodynamic parameters such as convective available potential energy (CAPE) and convective inhibition. In addition, the median value of surface-based CAPE (SBCAPE) was more than 2 times that of the mean-layer CAPE (MLCAPE). Thus, caution is advised when considering surface-based thermodynamic indices, despite the assumed presence of a well-mixed afternoon boundary layer. 1. Introduction dry-adiabatic temperature pro®le (constant potential The lifting condensation level (LCL) has long been temperature in the mixed layer) and a moisture pro®le used to estimate boundary layer cloud heights (e.g., described by a constant mixing ratio. -

Empirical Modelling of Einstein Absorption Refrigeration System
Journal of Advanced Research in Fluid Mechanics and Thermal Sciences 75, Issue 3 (2020) 54-62 Journal of Advanced Research in Fluid Mechanics and Thermal Sciences Journal homepage: www.akademiabaru.com/arfmts.html ISSN: 2289-7879 Empirical Modelling of Einstein Absorption Refrigeration Open Access System Keng Wai Chan1,*, Yi Leang Lim1 1 School of Mechanical Engineering, Engineering Campus, Universiti Sains Malaysia, 14300 Nibong Tebal, Penang, Malaysia ARTICLE INFO ABSTRACT Article history: A single pressure absorption refrigeration system was invented by Albert Einstein and Received 4 April 2020 Leo Szilard nearly ninety-year-old. The system is attractive as it has no mechanical Received in revised form 27 July 2020 moving parts and can be driven by heat alone. However, the related literature and Accepted 5 August 2020 work done on this refrigeration system is scarce. Previous researchers analysed the Available online 20 September 2020 refrigeration system theoretically, both the system pressure and component temperatures were fixed merely by assumption of ideal condition. These values somehow have never been verified by experimental result. In this paper, empirical models were proposed and developed to estimate the system pressure, the generator temperature and the partial pressure of butane in the evaporator. These values are important to predict the system operation and the evaporator temperature. The empirical models were verified by experimental results of five experimental settings where the power input to generator and bubble pump were varied. The error for the estimation of the system pressure, generator temperature and partial pressure of butane in evaporator are ranged 0.89-6.76%, 0.23-2.68% and 0.28-2.30%, respectively. -

Psychrometrics Outline
Psychrometrics Outline • What is psychrometrics? • Psychrometrics in daily life and food industry • Psychrometric chart – Dry bulb temperature, wet bulb temperature, absolute humidity, relative humidity, specific volume, enthalpy – Dew point temperature • Mixing two streams of air • Heating of air and using it to dry a product 2 Psychrometrics • Psychrometrics is the study of properties of mixtures of air and water vapor • Water vapor – Superheated steam (unsaturated steam) at low pressure – Superheated steam tables are on page 817 of textbook – Properties of dry air are on page 818 of textbook – Psychrometric charts are on page 819 & 820 of textbook • What are these properties of interest and why do we need to know these properties? 3 Psychrometrics in Daily Life • Sea breeze and land breeze – When and why do we get them? • How do thunderstorms, hurricanes, and tornadoes form? • What are dew, fog, mist, and frost and when do they form? • When and why does the windshield of a car fog up? – How do you de-fog it? Is it better to blow hot air or cold air? Why? • Why do you feel dry in a heated room? – Is the moisture content of hot air lower than that of cold air? • How does a fan provide relief from sweating? • How does an air conditioner provide relief from sweating? • When does a soda can “sweat”? • When and why do we “see” our breath? • Do sailboats perform better at high or low relative humidity? Key factors: Temperature, Pressure, and Moisture Content of Air 4 Do Sailboats Perform Better at low or High RH? • Does dry air or moist air provide more thrust against the sail? • Which is denser – humid air or dry air? – Avogadro’s law: At the same temperature and pressure, the no. -

Teaching Psychrometry to Undergraduates
AC 2007-195: TEACHING PSYCHROMETRY TO UNDERGRADUATES Michael Maixner, U.S. Air Force Academy James Baughn, University of California-Davis Michael Rex Maixner graduated with distinction from the U. S. Naval Academy, and served as a commissioned officer in the USN for 25 years; his first 12 years were spent as a shipboard officer, while his remaining service was spent strictly in engineering assignments. He received his Ocean Engineer and SMME degrees from MIT, and his Ph.D. in mechanical engineering from the Naval Postgraduate School. He served as an Instructor at the Naval Postgraduate School and as a Professor of Engineering at Maine Maritime Academy; he is currently a member of the Department of Engineering Mechanics at the U.S. Air Force Academy. James W. Baughn is a graduate of the University of California, Berkeley (B.S.) and of Stanford University (M.S. and PhD) in Mechanical Engineering. He spent eight years in the Aerospace Industry and served as a faculty member at the University of California, Davis from 1973 until his retirement in 2006. He is a Fellow of the American Society of Mechanical Engineering, a recipient of the UCDavis Academic Senate Distinguished Teaching Award and the author of numerous publications. He recently completed an assignment to the USAF Academy in Colorado Springs as the Distinguished Visiting Professor of Aeronautics for the 2004-2005 and 2005-2006 academic years. Page 12.1369.1 Page © American Society for Engineering Education, 2007 Teaching Psychrometry to Undergraduates by Michael R. Maixner United States Air Force Academy and James W. Baughn University of California at Davis Abstract A mutli-faceted approach (lecture, spreadsheet and laboratory) used to teach introductory psychrometric concepts and processes is reviewed. -

Thermodynamics of Interacting Magnetic Nanoparticles
This is a repository copy of Thermodynamics of interacting magnetic nanoparticles. White Rose Research Online URL for this paper: https://eprints.whiterose.ac.uk/168248/ Version: Accepted Version Article: Torche, P., Munoz-Menendez, C., Serantes, D. et al. (6 more authors) (2020) Thermodynamics of interacting magnetic nanoparticles. Physical Review B. 224429. ISSN 2469-9969 https://doi.org/10.1103/PhysRevB.101.224429 Reuse Items deposited in White Rose Research Online are protected by copyright, with all rights reserved unless indicated otherwise. They may be downloaded and/or printed for private study, or other acts as permitted by national copyright laws. The publisher or other rights holders may allow further reproduction and re-use of the full text version. This is indicated by the licence information on the White Rose Research Online record for the item. Takedown If you consider content in White Rose Research Online to be in breach of UK law, please notify us by emailing [email protected] including the URL of the record and the reason for the withdrawal request. [email protected] https://eprints.whiterose.ac.uk/ Thermodynamics of interacting magnetic nanoparticles P. Torche1, C. Munoz-Menendez2, D. Serantes2, D. Baldomir2, K. L. Livesey3, O. Chubykalo-Fesenko4, S. Ruta5, R. Chantrell5, and O. Hovorka1∗ 1School of Engineering and Physical Sciences, University of Southampton, Southampton SO16 7QF, UK 2Instituto de Investigaci´ons Tecnol´oxicas and Departamento de F´ısica Aplicada, Universidade de Santiago de Compostela,