Thermodynamics of Convection in the Moist Atmosphere B
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

How Does Convective Self-Aggregation Affect Precipitation Extremes?
EGU21-5216 https://doi.org/10.5194/egusphere-egu21-5216 EGU General Assembly 2021 © Author(s) 2021. This work is distributed under the Creative Commons Attribution 4.0 License. How does convective self-aggregation affect precipitation extremes? Nicolas Da Silva1, Sara Shamekh2, Caroline Muller2, and Benjamin Fildier2 1Centre for Ocean and Atmospheric Sciences, School of Environmental Sciences, University of East Anglia, Norwich, United Kingdom 2Laboratoire de Météorologie Dynamique (LMD)/Institut Pierre Simon Laplace (IPSL), École Normale Supérieure, Paris Sciences & Lettres (PSL) Research University, Sorbonne Université, École Polytechnique, CNRS, Paris, France Convective organisation has been associated with extreme precipitation in the tropics. Here we investigate the impact of convective self-aggregation on extreme rainfall rates. We find that convective self-aggregation significantly increases precipitation extremes, for 3-hourly accumulations but also for instantaneous rates (+ 30 %). We show that this latter enhanced instantaneous precipitation is mainly due to the local increase in relative humidity which drives larger accretion efficiency and lower re-evaporation and thus a higher precipitation efficiency. An in-depth analysis based on an adapted scaling of precipitation extremes, reveals that the dynamic contribution decreases (- 25 %) while the thermodynamic is slightly enhanced (+ 5 %) with convective aggregation, leading to lower condensation rates (- 20 %). When the atmosphere is more organized into a moist convecting region, and a dry convection-free region, deep convective updrafts are surrounded by a warmer environment which reduces convective instability and thus the dynamic contribution. The moister boundary-layer explains the positive thermodynamic contribution. The microphysic contribution is increased by + 50 % with aggregation. The latter is partly due to reduced evaporation of rain falling through a moister near-cloud environment (+ 30 %), but also to the associated larger accretion efficiency (+ 20 %). -

Soaring Weather
Chapter 16 SOARING WEATHER While horse racing may be the "Sport of Kings," of the craft depends on the weather and the skill soaring may be considered the "King of Sports." of the pilot. Forward thrust comes from gliding Soaring bears the relationship to flying that sailing downward relative to the air the same as thrust bears to power boating. Soaring has made notable is developed in a power-off glide by a conven contributions to meteorology. For example, soar tional aircraft. Therefore, to gain or maintain ing pilots have probed thunderstorms and moun altitude, the soaring pilot must rely on upward tain waves with findings that have made flying motion of the air. safer for all pilots. However, soaring is primarily To a sailplane pilot, "lift" means the rate of recreational. climb he can achieve in an up-current, while "sink" A sailplane must have auxiliary power to be denotes his rate of descent in a downdraft or in come airborne such as a winch, a ground tow, or neutral air. "Zero sink" means that upward cur a tow by a powered aircraft. Once the sailcraft is rents are just strong enough to enable him to hold airborne and the tow cable released, performance altitude but not to climb. Sailplanes are highly 171 r efficient machines; a sink rate of a mere 2 feet per second. There is no point in trying to soar until second provides an airspeed of about 40 knots, and weather conditions favor vertical speeds greater a sink rate of 6 feet per second gives an airspeed than the minimum sink rate of the aircraft. -

Chapter 8 Atmospheric Statics and Stability
Chapter 8 Atmospheric Statics and Stability 1. The Hydrostatic Equation • HydroSTATIC – dw/dt = 0! • Represents the balance between the upward directed pressure gradient force and downward directed gravity. ρ = const within this slab dp A=1 dz Force balance p-dp ρ p g d z upward pressure gradient force = downward force by gravity • p=F/A. A=1 m2, so upward force on bottom of slab is p, downward force on top is p-dp, so net upward force is dp. • Weight due to gravity is F=mg=ρgdz • Force balance: dp/dz = -ρg 2. Geopotential • Like potential energy. It is the work done on a parcel of air (per unit mass, to raise that parcel from the ground to a height z. • dφ ≡ gdz, so • Geopotential height – used as vertical coordinate often in synoptic meteorology. ≡ φ( 2 • Z z)/go (where go is 9.81 m/s ). • Note: Since gravity decreases with height (only slightly in troposphere), geopotential height Z will be a little less than actual height z. 3. The Hypsometric Equation and Thickness • Combining the equation for geopotential height with the ρ hydrostatic equation and the equation of state p = Rd Tv, • Integrating and assuming a mean virtual temp (so it can be a constant and pulled outside the integral), we get the hypsometric equation: • For a given mean virtual temperature, this equation allows for calculation of the thickness of the layer between 2 given pressure levels. • For two given pressure levels, the thickness is lower when the virtual temperature is lower, (ie., denser air). • Since thickness is readily calculated from radiosonde measurements, it provides an excellent forecasting tool. -

Effect of Wind on Near-Shore Breaking Waves
EFFECT OF WIND ON NEAR-SHORE BREAKING WAVES by Faydra Schaffer A Thesis Submitted to the Faculty of The College of Engineering and Computer Science in Partial Fulfillment of the Requirements for the Degree of Master of Science Florida Atlantic University Boca Raton, Florida December 2010 Copyright by Faydra Schaffer 2010 ii ACKNOWLEDGEMENTS The author wishes to thank her mother and family for their love and encouragement to go to college and be able to have the opportunity to work on this project. The author is grateful to her advisor for sponsoring her work on this project and helping her to earn a master’s degree. iv ABSTRACT Author: Faydra Schaffer Title: Effect of wind on near-shore breaking waves Institution: Florida Atlantic University Thesis Advisor: Dr. Manhar Dhanak Degree: Master of Science Year: 2010 The aim of this project is to identify the effect of wind on near-shore breaking waves. A breaking wave was created using a simulated beach slope configuration. Testing was done on two different beach slope configurations. The effect of offshore winds of varying speeds was considered. Waves of various frequencies and heights were considered. A parametric study was carried out. The experiments took place in the Hydrodynamics lab at FAU Boca Raton campus. The experimental data validates the knowledge we currently know about breaking waves. Offshore winds effect is known to increase the breaking height of a plunging wave, while also decreasing the breaking water depth, causing the wave to break further inland. Offshore winds cause spilling waves to react more like plunging waves, therefore increasing the height of the spilling wave while consequently decreasing the breaking water depth. -

Comparison Between Observed Convective Cloud-Base Heights and Lifting Condensation Level for Two Different Lifted Parcels
AUGUST 2002 NOTES AND CORRESPONDENCE 885 Comparison between Observed Convective Cloud-Base Heights and Lifting Condensation Level for Two Different Lifted Parcels JEFFREY P. C RAVEN AND RYAN E. JEWELL NOAA/NWS/Storm Prediction Center, Norman, Oklahoma HAROLD E. BROOKS NOAA/National Severe Storms Laboratory, Norman, Oklahoma 6 January 2002 and 16 April 2002 ABSTRACT Approximately 400 Automated Surface Observing System (ASOS) observations of convective cloud-base heights at 2300 UTC were collected from April through August of 2001. These observations were compared with lifting condensation level (LCL) heights above ground level determined by 0000 UTC rawinsonde soundings from collocated upper-air sites. The LCL heights were calculated using both surface-based parcels (SBLCL) and mean-layer parcels (MLLCLÐusing mean temperature and dewpoint in lowest 100 hPa). The results show that the mean error for the MLLCL heights was substantially less than for SBLCL heights, with SBLCL heights consistently lower than observed cloud bases. These ®ndings suggest that the mean-layer parcel is likely more representative of the actual parcel associated with convective cloud development, which has implications for calculations of thermodynamic parameters such as convective available potential energy (CAPE) and convective inhibition. In addition, the median value of surface-based CAPE (SBCAPE) was more than 2 times that of the mean-layer CAPE (MLCAPE). Thus, caution is advised when considering surface-based thermodynamic indices, despite the assumed presence of a well-mixed afternoon boundary layer. 1. Introduction dry-adiabatic temperature pro®le (constant potential The lifting condensation level (LCL) has long been temperature in the mixed layer) and a moisture pro®le used to estimate boundary layer cloud heights (e.g., described by a constant mixing ratio. -

NWS Unified Surface Analysis Manual
Unified Surface Analysis Manual Weather Prediction Center Ocean Prediction Center National Hurricane Center Honolulu Forecast Office November 21, 2013 Table of Contents Chapter 1: Surface Analysis – Its History at the Analysis Centers…………….3 Chapter 2: Datasets available for creation of the Unified Analysis………...…..5 Chapter 3: The Unified Surface Analysis and related features.……….……….19 Chapter 4: Creation/Merging of the Unified Surface Analysis………….……..24 Chapter 5: Bibliography………………………………………………….…….30 Appendix A: Unified Graphics Legend showing Ocean Center symbols.….…33 2 Chapter 1: Surface Analysis – Its History at the Analysis Centers 1. INTRODUCTION Since 1942, surface analyses produced by several different offices within the U.S. Weather Bureau (USWB) and the National Oceanic and Atmospheric Administration’s (NOAA’s) National Weather Service (NWS) were generally based on the Norwegian Cyclone Model (Bjerknes 1919) over land, and in recent decades, the Shapiro-Keyser Model over the mid-latitudes of the ocean. The graphic below shows a typical evolution according to both models of cyclone development. Conceptual models of cyclone evolution showing lower-tropospheric (e.g., 850-hPa) geopotential height and fronts (top), and lower-tropospheric potential temperature (bottom). (a) Norwegian cyclone model: (I) incipient frontal cyclone, (II) and (III) narrowing warm sector, (IV) occlusion; (b) Shapiro–Keyser cyclone model: (I) incipient frontal cyclone, (II) frontal fracture, (III) frontal T-bone and bent-back front, (IV) frontal T-bone and warm seclusion. Panel (b) is adapted from Shapiro and Keyser (1990) , their FIG. 10.27 ) to enhance the zonal elongation of the cyclone and fronts and to reflect the continued existence of the frontal T-bone in stage IV. -

Evidence of Fire in the Pliocene Arctic in Response to Elevated CO2 and Temperature
1 Evidence of fire in the Pliocene Arctic in response to elevated CO2 and temperature 2 Tamara Fletcher1*, Lisa Warden2*, Jaap S. Sinninghe Damsté2,3, Kendrick J. Brown4,5, Natalia 3 Rybczynski6,7, John Gosse8, and Ashley P Ballantyne1 4 1 College of Forestry and Conservation, University of Montana, Missoula, 59812, USA 5 2 Department of Marine Microbiology and Biogeochemistry, NIOZ Royal Netherlands Institute for Sea Research, Den 6 Berg, 1790, Netherlands 7 3 Department of Earth Sciences, University of Utrecht, Utrecht, 3508, Netherlands 8 4 Natural Resources Canada, Canadian Forest Service, Victoria, V8Z 1M, Canada 9 5 Department of Earth, Environmental and Geographic Science, University of British Columbia Okanagan, Kelowna, 10 V1V 1V7, Canada 11 6 Department of Palaeobiology, Canadian Museum of Nature, Ottawa, K1P 6P4, Canada 12 7 Department of Biology & Department of Earth Sciences, Carleton University, Ottawa, K1S 5B6, Canada 13 8 Department of Earth Sciences, Dalhousie University, Halifax, B3H 4R2, Canada 14 *Authors contributed equally to this work 15 Correspondence to: Tamara Fletcher ([email protected]) 16 Abstract. The mid-Pliocene is a valuable time interval for understanding the mechanisms that determine equilibrium 17 climate at current atmospheric CO2 concentrations. One intriguing, but not fully understood, feature of the early to 18 mid-Pliocene climate is the amplified arctic temperature response. Current models underestimate the degree of 19 warming in the Pliocene Arctic and validation of proposed feedbacks is limited by scarce terrestrial records of climate 20 and environment, as well as discrepancies in current CO2 proxy reconstructions. Here we reconstruct the CO2, summer 21 temperature and fire regime from a sub-fossil fen-peat deposit on west-central Ellesmere Island, Canada, that has been 22 chronologically constrained using radionuclide dating to 3.9 +1.5/-0.5 Ma. -

Downloaded 09/29/21 11:17 PM UTC 4144 MONTHLY WEATHER REVIEW VOLUME 128 Ferentiate Among Our Concerns About the Application of TABLE 1
DECEMBER 2000 NOTES AND CORRESPONDENCE 4143 The Intricacies of Instabilities DAVID M. SCHULTZ NOAA/National Severe Storms Laboratory, Norman, Oklahoma PHILIP N. SCHUMACHER NOAA/National Weather Service, Sioux Falls, South Dakota CHARLES A. DOSWELL III NOAA/National Severe Storms Laboratory, Norman, Oklahoma 19 April 2000 and 30 May 2000 ABSTRACT In response to Sherwood's comments and in an attempt to restore proper usage of terminology associated with moist instability, the early history of moist instability is reviewed. This review shows that many of Sherwood's concerns about the terminology were understood at the time of their origination. De®nitions of conditional instability include both the lapse-rate de®nition (i.e., the environmental lapse rate lies between the dry- and the moist-adiabatic lapse rates) and the available-energy de®nition (i.e., a parcel possesses positive buoyant energy; also called latent instability), neither of which can be considered an instability in the classic sense. Furthermore, the lapse-rate de®nition is really a statement of uncertainty about instability. The uncertainty can be resolved by including the effects of moisture through a consideration of the available-energy de®nition (i.e., convective available potential energy) or potential instability. It is shown that such misunderstandings about conditional instability were likely due to the simpli®cations resulting from the substitution of lapse rates for buoyancy in the vertical acceleration equation. Despite these valid concerns about the value of the lapse-rate de®nition of conditional instability, consideration of the lapse rate and moisture separately can be useful in some contexts (e.g., the ingredients-based methodology for forecasting deep, moist convection). -

MSE3 Ch14 Thunderstorms
Chapter 14 Copyright © 2011, 2015 by Roland Stull. Meteorology for Scientists and Engineers, 3rd Ed. thunderstorms Contents Thunderstorms are among the most violent and difficult-to-predict weath- Thunderstorm Characteristics 481 er elements. Yet, thunderstorms can be Appearance 482 14 studied. They can be probed with radar and air- Clouds Associated with Thunderstorms 482 craft, and simulated in a laboratory or by computer. Cells & Evolution 484 They form in the air, and must obey the laws of fluid Thunderstorm Types & Organization 486 mechanics and thermodynamics. Basic Storms 486 Thunderstorms are also beautiful and majestic. Mesoscale Convective Systems 488 Supercell Thunderstorms 492 In thunderstorms, aesthetics and science merge, making them fascinating to study and chase. Thunderstorm Formation 496 Convective Conditions 496 Thunderstorm characteristics, formation, and Key Altitudes 496 forecasting are covered in this chapter. The next chapter covers thunderstorm hazards including High Humidity in the ABL 499 hail, gust fronts, lightning, and tornadoes. Instability, CAPE & Updrafts 503 CAPE 503 Updraft Velocity 508 Wind Shear in the Environment 509 Hodograph Basics 510 thunderstorm CharaCteristiCs Using Hodographs 514 Shear Across a Single Layer 514 Thunderstorms are convective clouds Mean Wind Shear Vector 514 with large vertical extent, often with tops near the Total Shear Magnitude 515 tropopause and bases near the top of the boundary Mean Environmental Wind (Normal Storm Mo- layer. Their official name is cumulonimbus (see tion) 516 the Clouds Chapter), for which the abbreviation is Supercell Storm Motion 518 Bulk Richardson Number 521 Cb. On weather maps the symbol represents thunderstorms, with a dot •, asterisk , or triangle Triggering vs. Convective Inhibition 522 * ∆ drawn just above the top of the symbol to indicate Convective Inhibition (CIN) 523 Trigger Mechanisms 525 rain, snow, or hail, respectively. -

Potential Vorticity
POTENTIAL VORTICITY Roger K. Smith March 3, 2003 Contents 1 Potential Vorticity Thinking - How might it help the fore- caster? 2 1.1Introduction............................ 2 1.2WhatisPV-thinking?...................... 4 1.3Examplesof‘PV-thinking’.................... 7 1.3.1 A thought-experiment for understanding tropical cy- clonemotion........................ 7 1.3.2 Kelvin-Helmholtz shear instability . ......... 9 1.3.3 Rossby wave propagation in a β-planechannel..... 12 1.4ThestructureofEPVintheatmosphere............ 13 1.4.1 Isentropicpotentialvorticitymaps........... 14 1.4.2 The vertical structure of upper-air PV anomalies . 18 2 A Potential Vorticity view of cyclogenesis 21 2.1PreliminaryIdeas......................... 21 2.2SurfacelayersofPV....................... 21 2.3Potentialvorticitygradientwaves................ 23 2.4 Baroclinic Instability . .................... 28 2.5 Applications to understanding cyclogenesis . ......... 30 3 Invertibility, iso-PV charts, diabatic and frictional effects. 33 3.1 Invertibility of EPV ........................ 33 3.2Iso-PVcharts........................... 33 3.3Diabaticandfrictionaleffects.................. 34 3.4Theeffectsofdiabaticheatingoncyclogenesis......... 36 3.5Thedemiseofcutofflowsandblockinganticyclones...... 36 3.6AdvantageofPVanalysisofcutofflows............. 37 3.7ThePVstructureoftropicalcyclones.............. 37 1 Chapter 1 Potential Vorticity Thinking - How might it help the forecaster? 1.1 Introduction A review paper on the applications of Potential Vorticity (PV-) concepts by Brian -

Static Stability (I) the Concept of Stability (Ii) the Parcel Technique (A
ATSC 5160 – supplement: static stability Static stability (i) The concept of stability The concept of (local) stability is an important one in meteorology. In general, the word stability is used to indicate a condition of equilibrium. A system is stable if it resists changes, like a ball in a depression. No matter in which direction the ball is moved over a small distance, when released it will roll back into the centre of the depression, and it will oscillate back and forth, until it eventually stalls. A ball on a hill, however, is unstably located. To some extent, a parcel of air behaves exactly like this ball. Certain processes act to make the atmosphere unstable; then the atmosphere reacts dynamically and exchanges potential energy into kinetic energy, in order to restore equilibrium. For instance, the development and evolution of extratropical fronts is believed to be no more than an atmospheric response to a destabilizing process; this process is essentially the atmospheric heating over the equatorial region and the cooling over the poles. Here, we are only concerned with static stability, i.e. no pre-existing motion is required, unlike other types of atmospheric instability, like baroclinic or symmetric instability. The restoring atmospheric motion in a statically stable atmosphere is strictly vertical. When the atmosphere is statically unstable, then any vertical departure leads to buoyancy. This buoyancy leads to vertical accelerations away from the point of origin. In the context of this chapter, stability is used interchangeably -
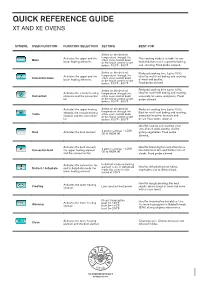
QUICK REFERENCE GUIDE XT and XE Ovens
QUICK REFERENCE GUIDE XT AND XE OVENS SYMBOL OVEN FUNCTION FUNCTION SELECTION SETTING BEST FOR Select on the desired Activates the upper and the temperature through the This cooking mode is suitable for any Bake either oven control knob lower heating elements or the touch control smart kind of dishes and it is great for baking botton. 100°F - 500°F and roasting. Food probe allowed. Select on the desired Reduced cooking time (up to 10%). Activates the upper and the temperature through the Ideal for multi-level baking and roasting Convection bake either oven control knob lower heating elements or the touch control smart of meat and poultry. botton. 100°F - 500°F Food probe allowed. Select on the desired Reduced cooking time (up to 10%). Activates the circular heating temperature through the Ideal for multi-level baking and roasting, Convection elements and the convection either oven control knob especially for cakes and pastry. Food fan or the touch control smart probe allowed. botton. 100°F - 500°F Activates the upper heating Select on the desired Reduced cooking time (up to 10%). temperature through the Best for multi-level baking and roasting, Turbo element, the circular heating either oven control knob element and the convection or the touch control smart especially for pizza, focaccia and fan botton. 100°F - 500°F bread. Food probe allowed. Ideal for searing and roasting small cuts of beef, pork, poultry, and for 4 power settings – LOW Broil Activates the broil element (1) to HIGH (4) grilling vegetables. Food probe allowed. Activates the broil element, Ideal for browning fish and other items 4 power settings – LOW too delicate to turn and thicker cuts of Convection broil the upper heating element (1) to HIGH (4) and the convection fan steaks.