12 Dec. Revisions 1 EXCEPTIONAL
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Cephalopoda) Diversity from Statolith Remains: Taxonomic Assignation, Fossil Record Analysis, and New Data for Calibrating Molecular Phylogenies
New Eocene coleoid (Cephalopoda) diversity from statolith remains: taxonomic assignation, fossil record analysis, and new data for calibrating molecular phylogenies. Pascal Neige, Hervé Lapierre, Didier Merle To cite this version: Pascal Neige, Hervé Lapierre, Didier Merle. New Eocene coleoid (Cephalopoda) diversity from sta- tolith remains: taxonomic assignation, fossil record analysis, and new data for calibrating molecu- lar phylogenies.. PLoS ONE, Public Library of Science, 2016, 11 (5), pp.e0154062. 10.1371/jour- nal.pone.0154062. hal-01319404 HAL Id: hal-01319404 https://hal.archives-ouvertes.fr/hal-01319404 Submitted on 23 May 2016 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Distributed under a Creative Commons Attribution| 4.0 International License RESEARCH ARTICLE New Eocene Coleoid (Cephalopoda) Diversity from Statolith Remains: Taxonomic Assignation, Fossil Record Analysis, and New Data for Calibrating Molecular Phylogenies Pascal Neige1*, Hervé Lapierre2, Didier Merle3 1 Univ. Bourgogne Franche-Comté, CNRS, Biogéosciences, 6 bd Gabriel, 21000, Dijon, France, a11111 2 Département Histoire de la Terre, (MNHN, CNRS, UPMC-Paris6), Paris, France, 3 Département Histoire de la Terre, Sorbonne Universités (CR2P—MNHN, CNRS, UPMC-Paris6), Paris, France * [email protected] Abstract OPEN ACCESS New coleoid cephalopods are described from statolith remains from the Middle Eocene Citation: Neige P, Lapierre H, Merle D (2016) New (Middle Lutetian) of the Paris Basin. -

Vita Scientia Revista De Ciências Biológicas Do CCBS
Volume III - Encarte especial - 2020 Vita Scientia Revista de Ciências Biológicas do CCBS © 2020 Universidade Presbiteriana Mackenzie Os direitos de publicação desta revista são da Universidade Presbiteriana Mackenzie. Os textos publicados na revista são de inteira responsabilidade de seus autores. Permite-se a reprodução desde que citada a fonte. A revista Vita Scientia está disponível em: http://vitascientiaweb.wordpress.com Dados Internacionais de Catalogação na Publicação (CIP) Vita Scientia: Revista Mackenzista de Ciências Biológi- cas/Universidade Presbiteriana Mackenzie. Semestral ISSN:2595-7325 UNIVERSIDADE PRESBITERIANA MACKENZIE Reitor: Marco Túllio de Castro Vasconcelos Chanceler: Robinson Granjeiro Pro-Reitoria de Graduação: Janette Brunstein Pro-Reitoria de Extensão e Cultura: Marcelo Martins Bueno Pro-Reitoria de Pesquisa e Pós-Graduação: Felipe Chiarello de Souza Pinto Diretora do Centro de Ciências Biológicas e da Saúde: Berenice Carpigiani Coordenador do Curso de Ciências Biológicas: Adriano Monteiro de Castro Endereço para correspondência Revista Vita Scientia Centro de Ciências Biológicas e da Saúde Universidade Presbiteriana Mackenzie Rua da Consolação 930, São Paulo (SP) CEP 01302907 E-mail: [email protected] Revista Vita Scientia CONSELHO EDITORIAL Adriano Monteiro de Castro Camila Sachelli Ramos Fabiano Fonseca da Silva Leandro Tavares Azevedo Vieira Patrícia Fiorino Roberta Monterazzo Cysneiros Vera de Moura Azevedo Farah Carlos Eduardo Martins EDITORES Magno Botelho Castelo Branco Waldir Stefano CAPA Bruna Araujo PERIDIOCIDADE Publicação semestral IDIOMAS Artigos publicados em português ou inglês Universidade Presbiteriana Mackenzie, Revista Vita Scientia Rua da Consolaçãoo 930, Edíficio João Calvino, Mezanino Higienópolis, São Paulo (SP) CEP 01302-907 (11)2766-7364 Apresentação A revista Vita Scientia publica semestralmente textos das diferentes áreas da Biologia, escritos em por- tuguês ou inglês: Artigo resultados científicos originais. -

Village Diary for January 4 Table Tennis 9
Village Diary for January 4 Table tennis 9 Luncheon Club / Parish Council 11 Mobile Library / Table Tennis 18 Probus / Women’s Institute 23 Luncheon Club 25 Table Tennis Church Services for January Sunday, 7th January Epiphany or Baptism of Christ 10.30am Holy Communion at Christian Malford Thursday, 11th January 9am Morning Prayer at Christian Malford Sunday, 14th January Epiphany 2 10.30am Coffee, Chat and Craft Christian Malford Sunday, 21st January Epiphany 3 10.30am United Benefice Holy Communion at Christian Malford Thursday, 25th January 9am Morning Prayer at Christian Malford Sunday, 28th January Epiphany 4 9am Holy Communion at Christian Malford Refuse collections for January Blue lid bin collections - Saturday 6th and Thursday 18th. Household waste, garden waste and black box collections Friday 12th and Thursday 25th To check your collection days visit: www.wiltshire.gov.uk/rubbish-collection-days Village Memorial Cross. Most days many villagers will walk past the memorial cross situated on The Green and perhaps cast a glance at the names of the servicemen carved thereon. Details of those servicemen and the circumstances in which they died will appear in these pages. Most died in the first world war and the brief notes that describe the circumstances in which they died bring home the sheer horror and often chaos of their situation. Private WILLIAM HENRY FREEGARD 202515, 2nd Battalion, Wiltshire Regiment who died on 8th May 1918 Son of Edward and Julia Freegard of 82 Thornend, Christian Malford, Wiltshire Remembered with honour at Tyne Cot Memorial William was son of a railway labourer living in the village. -

Lyneham and Bradenstoke Neighbourhood Development Plan 2016 to 2026 Draft June 2020
Lyneham and Bradenstoke Neighbourhood Development Plan 2016 to 2026 Draft June 2020 Table of Contents List of Figures i Glossary ii Foreword iii 1 Introduction 1 1.1 What is the Neighbourhood Plan? 1 1.2 Preparing the Plan 1 2 The Parish of Lyneham and Bradenstoke 3 2.1 History 3 2.1.1 Lyneham 3 2.1.2 Bradenstoke 4 2.1.3 Preston, Thickthorn and Woodside Cottages 5 2.2 Lyneham and Bradenstoke Today 6 2.2.1 Lyneham 6 2.2.2 Bradenstoke 7 2.2.3 Preston and Thickthorn 7 2.2.4 Woodside Cottages 8 3 Objectives 9 3.1 What Matters Most to our Community 9 3.2 Objectives 11 4 Strategic Aims 12 4.1 Housing Strategic Aims 12 4.2 Business, Employment and Services Strategic Aims 12 4.3 Leisure, Recreation and Open Space Strategic Aims 12 4.4 Getting Around Strategic Aims 12 5 Housing 13 5.1 Housing Strategic Aims 13 5.2 Housing Objectives 13 5.3 Housing - Context 13 Policy 1: Small Scale Residential Development 14 Policy 2: Design 14 6 Business, Employment and Services 16 6.1 Business, Employment & Services Strategic Aims 16 6.2 Business, Employment and Services Objectives 16 6.3 Business, Employment and Services Context 16 6.3.1 Business 16 6.3.2 Health Services 17 Policy 3: Brownfield Employment Development 17 Policy 4: Social and Medical Facilities 17 7 Leisure, Recreation and Open Space 18 7.1 Leisure, Recreation and Open Space Strategic Aims 18 7.2 Leisure, Recreation and Open Space Objectives 18 7.3 Leisure, Recreation and Open Spaces Context 20 Policy 5: Sports Facilities 21 Policy 6: Local Green Spaces 21 1. -

First Record of Non-Mineralized Cephalopod Jaws and Arm Hooks
Klug et al. Swiss J Palaeontol (2020) 139:9 https://doi.org/10.1186/s13358-020-00210-y Swiss Journal of Palaeontology RESEARCH ARTICLE Open Access First record of non-mineralized cephalopod jaws and arm hooks from the latest Cretaceous of Eurytania, Greece Christian Klug1* , Donald Davesne2,3, Dirk Fuchs4 and Thodoris Argyriou5 Abstract Due to the lower fossilization potential of chitin, non-mineralized cephalopod jaws and arm hooks are much more rarely preserved as fossils than the calcitic lower jaws of ammonites or the calcitized jaw apparatuses of nautilids. Here, we report such non-mineralized fossil jaws and arm hooks from pelagic marly limestones of continental Greece. Two of the specimens lie on the same slab and are assigned to the Ammonitina; they represent upper jaws of the aptychus type, which is corroborated by fnds of aptychi. Additionally, one intermediate type and one anaptychus type are documented here. The morphology of all ammonite jaws suggest a desmoceratoid afnity. The other jaws are identifed as coleoid jaws. They share the overall U-shape and proportions of the outer and inner lamellae with Jurassic lower jaws of Trachyteuthis (Teudopseina). We also document the frst belemnoid arm hooks from the Tethyan Maastrichtian. The fossils described here document the presence of a typical Mesozoic cephalopod assemblage until the end of the Cretaceous in the eastern Tethys. Keywords: Cephalopoda, Ammonoidea, Desmoceratoidea, Coleoidea, Maastrichtian, Taphonomy Introduction as jaws, arm hooks, and radulae are occasionally found Fossil cephalopods are mainly known from preserved (Matern 1931; Mapes 1987; Fuchs 2006a; Landman et al. mineralized parts such as aragonitic phragmocones 2010; Kruta et al. -

An Eocene Orthocone from Antarctica Shows Convergent Evolution of Internally Shelled Cephalopods
RESEARCH ARTICLE An Eocene orthocone from Antarctica shows convergent evolution of internally shelled cephalopods Larisa A. Doguzhaeva1*, Stefan Bengtson1, Marcelo A. Reguero2, Thomas MoÈrs1 1 Department of Palaeobiology, Swedish Museum of Natural History, Stockholm, Sweden, 2 Division Paleontologia de Vertebrados, Museo de La Plata, Paseo del Bosque s/n, B1900FWA, La Plata, Argentina * [email protected] a1111111111 a1111111111 a1111111111 a1111111111 Abstract a1111111111 Background The Subclass Coleoidea (Class Cephalopoda) accommodates the diverse present-day OPEN ACCESS internally shelled cephalopod mollusks (Spirula, Sepia and octopuses, squids, Vampyro- teuthis) and also extinct internally shelled cephalopods. Recent Spirula represents a unique Citation: Doguzhaeva LA, Bengtson S, Reguero MA, MoÈrs T (2017) An Eocene orthocone from coleoid retaining shell structures, a narrow marginal siphuncle and globular protoconch that Antarctica shows convergent evolution of internally signify the ancestry of the subclass Coleoidea from the Paleozoic subclass Bactritoidea. shelled cephalopods. PLoS ONE 12(3): e0172169. This hypothesis has been recently supported by newly recorded diverse bactritoid-like doi:10.1371/journal.pone.0172169 coleoids from the Carboniferous of the USA, but prior to this study no fossil cephalopod Editor: Geerat J. Vermeij, University of California, indicative of an endochochleate branch with an origin independent from subclass Bactritoi- UNITED STATES dea has been reported. Received: October 10, 2016 Accepted: January 31, 2017 Methodology/Principal findings Published: March 1, 2017 Two orthoconic conchs were recovered from the Early Eocene of Seymour Island at the tip Copyright: © 2017 Doguzhaeva et al. This is an of the Antarctic Peninsula, Antarctica. They have loosely mineralized organic-rich chitin- open access article distributed under the terms of compatible microlaminated shell walls and broadly expanded central siphuncles. -
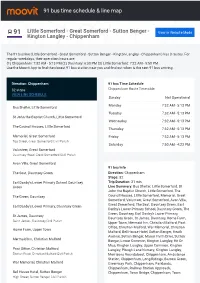
91 Bus Time Schedule & Line Route
91 bus time schedule & line map 91 Little Somerford - Great Somerford - Sutton Benger - View In Website Mode Kington Langley - Chippenham The 91 bus line (Little Somerford - Great Somerford - Sutton Benger - Kington Langley - Chippenham) has 3 routes. For regular weekdays, their operation hours are: (1) Chippenham: 7:32 AM - 5:13 PM (2) Dauntsey: 6:38 PM (3) Little Somerford: 7:22 AM - 5:50 PM Use the Moovit App to ƒnd the closest 91 bus station near you and ƒnd out when is the next 91 bus arriving. Direction: Chippenham 91 bus Time Schedule 32 stops Chippenham Route Timetable: VIEW LINE SCHEDULE Sunday Not Operational Monday 7:32 AM - 5:13 PM Bus Shelter, Little Somerford Tuesday 7:32 AM - 5:13 PM St John the Baptist Church, Little Somerford Wednesday 7:32 AM - 5:13 PM The Council Houses, Little Somerford Thursday 7:32 AM - 5:13 PM Memorial, Great Somerford Friday 7:32 AM - 5:13 PM Top Street, Great Somerford Civil Parish Saturday 7:50 AM - 4:23 PM Volunteer, Great Somerford Dauntsey Road, Great Somerford Civil Parish Avon Villa, Great Somerford 91 bus Info The Seat, Dauntsey Green Direction: Chippenham Stops: 32 Earl Danby's Lower Primary School, Dauntsey Trip Duration: 31 min Green Line Summary: Bus Shelter, Little Somerford, St John the Baptist Church, Little Somerford, The The Green, Dauntsey Council Houses, Little Somerford, Memorial, Great Somerford, Volunteer, Great Somerford, Avon Villa, Great Somerford, The Seat, Dauntsey Green, Earl Earl Danby's Lower Primary, Dauntsey Green Danby's Lower Primary School, Dauntsey Green, The -

CM NDP (Consultation)
CHRISTIAN MALFORD NEIGHBOURHOOD DEVELOPMENT PLAN 2015-2035 1773 ca 2005 For Consultation CONTENTS Foreword Section 1: Introduction and Section 7: Employment and Background Business Policies 1.1 Purpose 7.1 Business 1.2 Submitting Body 1.3 Neighbourhood Area Section 8: Countryside and 1.4 The Context Environmental Policies 1.5 Plan Period, Monitoring and Review 1.6 Existing Planning Policy 8.1 Rural Look and Feel 8.2 The Historic Environment Section 2: Process Summary Section 9: Housing Policies 2.1 Plan Development Process 2.2 Community Engagement 9.1 Background 2.3 Evidence base overview 9.2 Housing Strategy 9.3 Numbers of New Dwellings Section 3: Goals and Objectives 9.4 Size of New Developments 9.5 Tenancy of Homes 3.1 Vision 9.6 Affordable Homes for Local People 3.2 Goals 9.7 New Homes – Type and Size 3.3 Plan Objectives 9.8 Retirement Housing Provision Section 4: Christian Malford – Section 10: Design Policies Our Village 10.1 Design 4.1 Location & Connections 4.2 Landscape Section 11: Housing Sites Policies 4.3 Heritage 4.4 Population 11.1 Site Allocations 4.5 The Natural Environment 11.2 Delivery and Contingency 4.6 Housing 11.3 Sites for which planning permission will be supported. 11.4 Impact of the proposals on the Section 5: Community Well- Historic Assets of the Parish being Policies 11.5 Development Site Details 5.1 Community and Recreational Facilities 5.2 Health and Health Care APPENDICES 5.3 Communications Infrastructure Appendix A Glossary 5.4 Facilities Appendix B Location Assessment 5.5 Education Appendix C Summary -

Kington Langley Village Magazine January 2019
Kington Langley and Draycot Cerne Village Magazine January 2019 Issue no. 473 Draycot Benefice Services for January 2019 th Sunday 6th January Sunday 20 January Epiphany 1 Epiphany 3 9am Holy Communion at Seagry 9am Holy Communion (BCP) at Tytherton 10.30am Holy Communion at Christian Kellaways Malford 10.30am United Benefice Holy Communion 10.30am All Age Service at Kington at Kington Langley Langley 10.30 Café Church at Sutton Benger Thursday 24th January 9am Morning Prayer at Christian Malford Thursday 10th January 9am Morning Prayer at Christian Malford Sunday 27th January Sunday 13th January Epiphany 4 Epiphany 2 or Baptism of Christ 9am Holy Communion at Christian Malford 9am Holy Communion at Kington Langley 10.30am Holy Communion at Kington Langley 10.30am Holy Communion and Plough Sunday at Sutton Benger 10.30am All Age Service at Sutton Benger 10.30am Coffee, Chat and Craft Christian Malford Thursday 17th January 9am Holy Communion at Kington Langley Union Chapel Christian Fellowship – Kington Langley SERVICES & EVENTS Sunday 6th January 10.30am Morning Service Sunday 13th January 10.30am Morning Service Sunday 20th January 10.30am Morning Service Sunday 27th January 10.30am Morning Service Weekly events Thursdays 10.00 - 1200 Chapel Rendezvous in the Chapel FROM THE EDITOR Welcome to the first edition of 2019 – January is not an easy month for many Happy New Year! I hope you had a peaceful people – as the article for the Samaritans and enjoyable festive season. By the time highlights, 21st January has been dubbed you receive this magazine there may still be ‘the most difficult day of the year’. -

60 and 95 in the Brinkworth, Bradenstoke, Christian Malford, Foxham, Bremhill and Langley Burrell Areas We Want Your Views
Proposed changes to ‘shoppers buses’ 60 and 95 in the Brinkworth, Bradenstoke, Christian Malford, Foxham, Bremhill and Langley Burrell areas We want your views ! Wiltshire Council is reviewing the bus services it funds in the north of the county, with the aim of providing them in a more cost effective manner whilst continuing to meet local needs. Bus services 60 and 95 are funded entirely by Wiltshire Council and currently run as follows: Service 60 – runs to Wootton Bassett and Swindon on Fridays only from Castle Combe, Yatton Keynell, Biddestone, Cepen Park, Kington Langley, Sutton Benger, Christian Malford and Bradenstoke, arriving in Swindon at 10.30 am and returning from there at 1.30 pm. Service 95 – runs to Chippenham every day (except Sundays), from Brinkworth, Bradenstoke, Foxham, Bremhill and Langley Burrell, arriving in Chippenham at 10.10 am and returning at 12.15 pm on weekdays and 1.15 pm on Saturdays. Parts of these services are poorly used, and so this consultation is being used to identify the needs that the current services meet, so that we can look, with the help of local communities, for better and more affordable ways of meeting these needs in the future. If you use either of these services, please complete the questionnaire overleaf and return it to us at the address shown, by 7 October 2013. Possible options for providing these bus services in the future Both of these services are mainly used by residents of Bradenstoke, with service 95 also being used on certain days by passengers from Bremhill and Langley Burrell. -

Evidence for Fish Predation on a Coleoid Cephalopod from the Lower Jurassic Posidonia Shale of Germany
N. Jb. Geol. Paläont. Abh. 263/1, 25 – 33 Article Stuttgart, January 2012 Evidence for fish predation on a coleoid cephalopod from the Lower Jurassic Posidonia Shale of Germany Tomáš Přikryl, Martin Košťák, Martin Mazuch, and Radek Mikuláš With 4 figures Přikryl, T., košT’ák, M., Mazuch, M. & Mikuláš, R. (2012): Evidence for fish predation on a coleoid cephalopod from the Lower Jurassic Posidonia Shale of Germany. – N. Jb. Geol. Paläont. Abh., 263: 25-33; Stuttgart. Abstract: A specimen of the Early Jurassic actinopterygian fish Pachycormus sp. from the Lower Jurassic Posidonia Shale of Germany has a well preserved filling of the alimentary canal. The region interpreted as the stomach contains numerous hooklets that can be referred to the coleoid cephalopod Phragmoteuthis Mojsisovics, 1882. The presence of arm hooklets clearly demonstrates predation on coleoid cephalopods by actinopterygian fishes. Key words: Taphonomy, Cephalopoda, Actinopterygia, predation, Jurassic, Germany. 1. Introduction 1.1. Palaeoecological setting A specimen of the actinopterygian fish Pachycormus The Lower Jurassic (Toarcian) Posidonia Shale fauna sp. (IGP 163/1881) in the collections of the Institute is famous for the exceptional preservation of crinoids, of Geology and Palaeontology, Faculty of Science, crustaceans, cephalopods, fish, sharks, ichthyosaurs, Charles University in Prague, is notable for the crocodiles and other marine reptiles (nudds & preservation of stomach contents in the lumen of the selden 2008) and, as such, represents a marine fossil gut. Pachycomids were large to very large pelagic Konservat Lagerstätten. predators typified by fusiform bodies, a prominent rostrum, sickle-shaped pectoral fins and a deeply 2. Material and methods forked caudal fin. -

'VILTSHIRE. [KELLY's Walmesley John Esq
60 CHIPP~HAM. 'VILTSHIRE. [KELLY'S Walmesley John esq. Lucknam, Colerne, Chippenham For Bankruptcy purposes this Court is included in that Ex-{)fficio, the Mayor of Chippenham of Bath, F. Clarke, Bank chambers, Oorn st. Bristol. Clerk tD the Magistrates, George Alfred Huelin White, official receiver High street Certified Bailiffs under the "Law of Distress Amend Petty Sessions are held at the new Hall, Chippenham, on ment Act," Charles Brent Pollard, Cook stre·et, Chip the Ist thursday &i on the 3rd thursday in the month, penham; Thomas C. Parry, Chippenham; & William at the Town hall, Oorsham, at II.30 a.m. The follow 'feagle, Market place, Chippenham ing places are included in the petty sessional divi Cottage Hospital (W. T. Briscoe B.A., M.D., M.Ch. & sion :-Alderton, .A1lington, Avon, Biddestone, Box, M. S. Wilson RA. Camb. , l\'LRC.S.Eng. hon. medical Oastle Combe, Chippenham, Christian Mallard, Colerne, officers; F. K. Green F.R. C.S. hon. consulting sur Corsham, Ditteridge, Draycot, Cerne, Grittleton, geon; W. Helyar L.D.S. hon. dental surgeon; E. M. Hardenhuish, Kington St. Michael, Lacock, Lang. Awdry, hon. sec.; L. H. Marshall, hon. treas.; :Miss ley Fitzurse or Kington Langley, West Kington, Lang E. Stevens, matTon) ley Burrell, Leigh Delamere, Littleton Drew or Little County Police Station, Kew road, John Duly, supt.; ton St. Andrew, Nettleton, Pewsham, SeagTy, Slaugh Hubert Watel's, sergeant &; 3 constables terford, Sbanley, Stanton St. Quinton, Sutton Benger, Fire Brigade, Market place, A. E. Adams, snpt.; H. G. Tytherton Kelways (Kellaway.s or Cailoes), Tytherton Phipps, chief officer, & 12 men Lucas, North Wraxhall &i Yatton Keynell Inland Revenue Offices, 5 High street, John Pattcr"'lQn.