Not for Reuse Or Distribution --- 8
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
W7192e19.Pdf
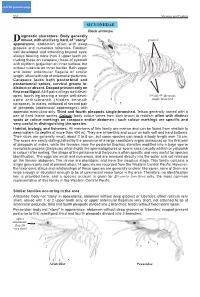
click for previous page 952 Shrimps and Prawns Sicyoniidae SICYONIIDAE Rock shrimps iagnostic characters: Body generally Drobust, with shell very hard, of “stony” grooves appearance; abdomen often with deep grooves and numerous tubercles. Rostrum well developed and extending beyond eyes, always bearing more than 3 upper teeth (in- cluding those on carapace); base of eyestalk with styliform projection on inner surface, but without tubercle on inner border. Both upper and lower antennular flagella of similar length, attached to tip of antennular peduncle. 1 Carapace lacks both postorbital and postantennal spines, cervical groove in- distinct or absent. Exopod present only on first maxilliped. All 5 pairs of legs well devel- 2 oped, fourth leg bearing a single well-devel- 3rd and 4th pleopods 4 single-branched oped arthrobranch (hidden beneath 3 carapace). In males, endopod of second pair 5 of pleopods (abdominal appendages) with appendix masculina only. Third and fourth pleopods single-branched. Telson generally armed with a pair of fixed lateral spines. Colour: body colour varies from dark brown to reddish; often with distinct spots or colour markings on carapace and/or abdomen - such colour markings are specific and very useful in distinguishing the species. Habitat, biology, and fisheries: All members of this family are marine and can be found from shallow to deep waters (to depths of more than 400 m). They are all benthic and occur on both soft and hard bottoms. Their sizes are generally small, about 2 to 8 cm, but some species can reach a body length over 15 cm. The sexes are easily distinguished by the presence of a large copulatory organ (petasma) on the first pair of pleopods of males, while the females have the posterior thoracic sternites modified into a large sperm receptacle process (thelycum) which holds the spermatophores or sperm sacs (usually whitish or yellowish in colour) after mating. -

WSR Vol 6 for 508 Pdf.Indd
Coastal and Estuarine Hazardous Waste Site Reports Editors J. Gardiner1, B. Azzato2, M. Jacobi1 1NOAA/OR&R/Coastal Protection and Restoration Division 2Azzato Communications Authors M. Hilgart, S. Duncan, S. Pollock Ridolfi Engineers Inc. NOAA National Oceanic and Atmospheric Administration NOS NOAA’s Ocean Service OR&R Office of Response and Restoration CPRD Coastal Protection and Restoration Division 7600 Sand Point Way NE Seattle, Washington 98115 September 30, 2004 Coastal and Estuarine Hazardous Waste Site Reports Reviewers K. Finkelstein1, M. Geddes2, M. Gielazyn1, R. Gouguet1, R. Mehran1 1NOAA/OR&R/Coastal Protection and Restoration Division 2Genwest Systems Graphics K. Galimanis 4 Point Design NOAA National Oceanic and Atmospheric Administration NOS NOAA’s Ocean Service OR&R Office of Response and Restoration CPRD Coastal Protection and Restoration Division 7600 Sand Point Way NE Seattle, Washington 98115 September 30, 2004 PLEASE CITE AS: J. Gardiner, B. Azzato and M. Jacobi, editors. 2004. Coastal and Estuarine Hazardous Waste Site Reports, September 30, 2004. Seattle: Coastal Protection and Restoration Division, Office of Response and Restoration, National Oceanic and Atmospheric Administration. 130 pp. v Contents Acronyms and abbreviations vii Introduction ix EPA Region 1 Callahan Mining Corp 1 Brooksville (Cape Rosier), Maine EPA Region 2 Diamond Head Oil Refinery Div. 11 Kearny, New Jersey MacKenzie Chemical Works 21 Central Islip, New York Pesticide Warehouse III 31 Manatí, Puerto Rico EPA Region 4 Davis Timber Company 41 Hattiesburg, -

Universidad De Costa Rica Facultad De Ciencias Escuela De Biologia
UNIVERSIDAD DE COSTA RICA FACULTAD DE CIENCIAS ESCUELA DE BIOLOGIA Optando por el grado académico de Licenciatura en Biología con énfasis en Recursos Acuáticos Morfometría y reproducción de tres especies langostinos de la vertiente del Pacífico de Costa Rica: Macrobrachium panamense, M. americanum y M. tenellum (Decapoda: Palaemonidae). Yurlandy Gutiérrez Jara Cédula 1-1057-0627 Carné: 985134 Miembros del comité Dr. Ingo Wehrtmann (Director de Tesis) M.Sc. Gerardo Umaña (Lector) M.Sc. Monika Springer (Lectora) MIEMBROS DEL COMITÉ REVISOR Firma: __________________________ Dr. Ingo Wehrtmann Director de Tesis Firma: __________________________ M.Sc. Gerardo Umaña Lector Firma: __________________________ M.Sc. Monika Springer Lectora Firma: __________________________ Dra. Virginia Solís Alvarado Presidenta del tribunal Firma: __________________________ Dr. Paul Hanson Revisor Externo Firma: __________________________ Biol. Yurlandy Gutiérrez Jara Postulante II Este trabajo esta dedicado con todo mi amor: a mi esposo Rólier Lara y mi hermoso hijo Matias Lara Gutiérrez A mi madre Virginia Jara Mis hermanas: Montserrath, Daniela y a mi sobrina Sophi Y con mucho cariño a mi hermana mayor Layin que desde el cielo siempre me cuida y guía TQM. III AGRADECIMIENTOS Agradezco a los profesores: Ingo por su ayuda y guía en el desarrollo de mi tesis. A Monika por sus valiosas sugerencias y a Don Gerardo por su colaboración, apoyo y formación en trabajo de campo. Además a todas las secres de Biología por su apoyo. A Jeffrey Sibaja por su guía en la utilización del programa estadístico, para la elaboración de pruebas. A la empresa Rainbow por su aporte económico en la logística del trabajo de campo, compra de equipo y viáticos utilizados. -

Puerto Rico Comprehensive Wildlife Conservation Strategy 2005
Comprehensive Wildlife Conservation Strategy Puerto Rico PUERTO RICO COMPREHENSIVE WILDLIFE CONSERVATION STRATEGY 2005 Miguel A. García José A. Cruz-Burgos Eduardo Ventosa-Febles Ricardo López-Ortiz ii Comprehensive Wildlife Conservation Strategy Puerto Rico ACKNOWLEDGMENTS Financial support for the completion of this initiative was provided to the Puerto Rico Department of Natural and Environmental Resources (DNER) by U.S. Fish and Wildlife Service (USFWS) Federal Assistance Office. Special thanks to Mr. Michael L. Piccirilli, Ms. Nicole Jiménez-Cooper, Ms. Emily Jo Williams, and Ms. Christine Willis from the USFWS, Region 4, for their support through the preparation of this document. Thanks to the colleagues that participated in the Comprehensive Wildlife Conservation Strategy (CWCS) Steering Committee: Mr. Ramón F. Martínez, Mr. José Berríos, Mrs. Aida Rosario, Mr. José Chabert, and Dr. Craig Lilyestrom for their collaboration in different aspects of this strategy. Other colleagues from DNER also contributed significantly to complete this document within the limited time schedule: Ms. María Camacho, Mr. Ramón L. Rivera, Ms. Griselle Rodríguez Ferrer, Mr. Alberto Puente, Mr. José Sustache, Ms. María M. Santiago, Mrs. María de Lourdes Olmeda, Mr. Gustavo Olivieri, Mrs. Vanessa Gautier, Ms. Hana Y. López-Torres, Mrs. Carmen Cardona, and Mr. Iván Llerandi-Román. Also, special thanks to Mr. Juan Luis Martínez from the University of Puerto Rico, for designing the cover of this document. A number of collaborators participated in earlier revisions of this CWCS: Mr. Fernando Nuñez-García, Mr. José Berríos, Dr. Craig Lilyestrom, Mr. Miguel Figuerola and Mr. Leopoldo Miranda. A special recognition goes to the authors and collaborators of the supporting documents, particularly, Regulation No. -

Embryonic Development of the Palaemonid Prawn Macrobrachium Idella Idella (Hilgendorf, 1898) G
lopmen ve ta e l B D Dinakaran et al., Cell Dev Biol 2013, 2:1 io & l l o DOI: 10.4172/2168-9296.1000111 l g e y C Cell & Developmental Biology ISSN: 2168-9296 Research Article Open Access Embryonic Development of the Palaemonid Prawn Macrobrachium idella idella (Hilgendorf, 1898) G. K. Dinakaran, P. Soundarapandian* and D. Varadharajan Faculty of Marine Sciences, Centre of Advanced Study in Marine Biology, Annamalai University, India Abstract After mating, the eggs were deposited, or oviposited, on setae of the pleopods of the female. The newly oviposited eggs were containing all the necessary material for synthetic processes associated with embryogenesis and morphogenesis and all the compounds required for oxidative metabolism and energy production. The fertilized eggs were opaque, greenish, round or oval in shape. The diameter of the egg was approximately 0.45 mm. As the development progresses, the greenish colour changed into light green, brownish-yellow and finally to dull whitish in colour about to hatch. The incubation periods varied from 12-14 days. The process of embryonic development includes nuclear division, cleavage (blastomeres), segmentation, formation of optic vesicle, eye pigment development and larva formation. At third minute after mating the sperm fused with the egg membrane and subsequently the male pronucleus entered the egg’s cytoplasm. The first and second nuclear divisions were completed without any corresponding division of the cell. Third division begun at 8 h and eight nuclei were formed after 9 h. Subsequent divisions of sixteen and thirty two nuclei stage took place at about 1 to 1.30 h interval and segmentation was completed at 18-20h. -

Aquaculture of Fresh Water Prawns/Macrobrachium Species
0804 quaculture of Fresh Water Prawns/Macrobrachium Species THE OCEANIC INSTITUTE/Waima.. nrB.lo, Hawaii DISCLAIMER This report was prepared as an account of work sponsored by an agency of the United States Government. Neither the United States Government nor any agency Thereof, nor any of their employees, makes any warranty, express or implied, or assumes any legal liability or responsibility for the accuracy, completeness, or usefulness of any information, apparatus, product, or process disclosed, or represents that its use would not infringe privately owned rights. Reference herein to any specific commercial product, process, or service by trade name, trademark, manufacturer, or otherwise does not necessarily constitute or imply its endorsement, recommendation, or favoring by the United States Government or any agency thereof. The views and opinions of authors expressed herein do not necessarily state or reflect those of the United States Government or any agency thereof. DISCLAIMER Portions of this document may be illegible in electronic image products. Images are produced from the best available original document. ,o~ Goodwin & Goodwin photo Dr. Shao-wen Ling, first scientist to control the life cycle of Macrobrachium rosenbergii, is also an artist. He painted "Malaysian Prawns",. used as the cover of this publication with his permission, to commemorate the first prawn culture workshop at St. Petersburg, Florida, in November, 1974. Dr. Ling holds a fine example of Macrobrachium rosenbergii brood stock selected from a King Caribe Shrimp brood pond near Cabo Rojo, Puerto Rico. The photo was taken during the 4th Food and Drugs from the Sea Conference, held at Mayaguez, in November, 1974. -

LIBRO ROJO De La Fauna Venezolana 4Ta Edición 2015 Jon Paul Rodríguez Ariany García-Rawlins Franklin Rojas-Suárez
LIBRO ROJO DE LA fAUNA vENEZOLANA 4ta edición 2015 Jon Paul Rodríguez Ariany García-Rawlins Franklin Rojas-Suárez Selección de Especies ubicadas en el estado Lara 1 Ángel del sol de Mérida / EN Heliangelus spencei Javier Mesa 2 Créditos Editores Autores Jürg De Marmels Romina Acevedo Jon Paul Rodríguez Abraham Mijares-Urrutia Dorixa Monsalve Douglas Rodríguez-Olarte Kareen De Turris-Morales Salvador Boher-Bentti Ariany M. García-Rawlins Ada Sánchez-Mercado Adda G. Manzanilla Fuentes Edgard Yerena Kathryn Rodríguez-Clark Samuel Narciso Franklin Rojas-Suárez Ahyran Amaro Eliane García Lenín Oviedo Shaenandhoa García-Rangel Ainhoa L. Zubillaga Eliécer E. Gutiérrez Leonardo Sánchez-Criollo Sheila Márques Pauls Editores Asociados Aldo Cróquer Emiliana Isasi-Catalá Lucy Perera Sofía Marín Wikander Mamíferos Alfredo Arteaga Eneida Marín Luis Bermúdez-Villapol Tatiana Caldera Daniel Lew Alimar Molero-Lizarraga Enrique La Marca Manuel Ruiz-Garcí Tatiana León Javier Sánchez Alma R. Ulloa Ernesto O. Boede Marcela Portocarrero-Aya Tito Barros Aves Ana Carolina Peralta Ernesto Ron Marcial Quiroga-Carmona Vicente J. Vera Christopher Sharpe Ana Iranzo Estrella Villamizar Marco Antonio García Cruz Víctor Pacheco Marcos A. Campo Z. Víctor Romero Miguel Lentino Andrés E. Seijas Ezequiel Hidalgo Fátima I. Lameda-Camacaro Margenny Barrios William P. McCord Reptiles Andrés Eloy Bracho Andrés Orellana Fernando Rojas-Runjaic María Alejandra Esteves Wlodzimierz Jedrzejewski Andrés E. Seijas Ángel L. Viloria Fernando Trujillo María Alejandra Faría Romero Yelitza Rangel César Molina † Aniello Barbarino Francisco Bisbal María de los Á. Rondón-Médicci Hedelvy Guada Antonio J. González-Fernández Francisco Provenzano María Fernanda Puerto Carrillo Ilustradores Omar Hernández Antonio Machado-Allison Franger J. -

In Non-Vegetated Areas of Two River Islands in a Brazilian Amazon Estuary
ZOOLOGIA 30 (6): 652–660, December, 2013 http://dx.doi.org/10.1590/S1984-46702013005000004 Composition of shrimp populations (Crustacea: Decapoda) in non-vegetated areas of two river islands in a Brazilian Amazon estuary Priscila Sousa Vilela da Nóbrega1,3, Bianca Bentes2 & Jussara Moretto Martinelli-Lemos1 1 Laboratório de Biologia Pesqueira e Manejo dos Recursos Aquáticos, Grupo de Pesquisa em Ecologia de Crustáceos da Amazônia, Instituto de Ciências Biológicas, Universidade Federal do Pará. Avenida Perimetral 2651, Montese, 66077-530 Belém, PA, Brazil. 2 Instituto de Estudos Costeiros, Universidade Federal do Pará. Alameda Leandro Ribeiro, 68600-000 Bragança, PA, Brazil. 3 Corresponding author. E-mail: [email protected] ABSTRACT. This study investigates the shrimp found in non-vegetated areas of an estuary of the Amazon River. We ascertained the input of juveniles, species’ biometrics and the influence of environmental factors on the abundance of species. The samples were collected monthly, from August 2006 to July 2007, in two places in the estuary, each next to an island. For collecting, we used a manual trawl to perform three hauls per month, totaling 36 samples per site. The abundance of shrimps was estimated as a function of the density of specimens per unit area. We used the Spearman’s correlation to test the hypothesis that there is significant correlation between the average of the environmental vari- ables measured and variations in shrimp density. The Kruskal-Wallis and the Mann-Whitney tests showed that there were significant differences in environment factors (temperature and salinity) among the months and sites. We ob- tained 6,091 shrimps, from which 5,231 (85.88%) were caught off the Arapiranga Island and 860 (14.12%) off the Mosqueiro Island, Palaemonidae and Penaeidae were the only families recorded. -

Lemmon, Julia (2004) Quick-Reference Pictorial Key to the Prawns of the Check Hall River
Quick-Reference Pictorial Key to the Prawns of the Check Hall River Julia Lemmon Dominica 2004 May 28 - June 17 1 Abstract This key to prawns of the Check Hall River was constructed to create a simple, step-by-step, pictorial guide to show the characteristics that differentiate one prawn species from another. There are certain body parts that one needs to inspect when identifying prawns, and these have been pictured and labeled in this key. Descriptive behavioral characteristics are also mentioned for the three genera of prawns, along with other general observations about their location and activity in the river. Introduction Prawns are freshwater decapods that are found in rivers and streams. They can easily be compared to the well-known saltwater shrimp when discussing general morphology. Unlike saltwater shrimp, prawns have two pair of pinchers, with some species possessing very long, large pinchers on the second pereiopod. This adaptive trait allows prawns to reach into deep cracks and holes between rocks (Elovaara, 2002). The pinchers also provide an effective means of defense towards predators. Prawns do not exit the water and can be found at all times of the day. However, prawns can be more easily spotted after dark when they swim out from behind rocks and other organic matter, which provides them protection from light throughout the day. Prawns are scavengers that feed on many things that float through the river, such as coconut, smaller prawns, worms, insect larva, etc (Elovaara, 2002). Kingdom: Animalia Phylum: Arthropoda Class: Crustacea Order: Decopoda 2 The Check Hall River is located on the southwest side of the West Indies Island of Dominica. -

Thesis Body-No Codes-Final
The plasticity of life histories during larval development and metamorphosis, using amphibians as study organisms. Patrick Thomas Walsh Presented for the degree of Doctor of Philosophy Division of Environmental and Evolutionary Biology Institute of Biomedical and Life Sciences University of Glasgow December 2007 ©Patrick Thomas Walsh 2007 Candidate’s declaration I declare that the work presented in this study is entirely my own unless otherwise stated and that it is of my own composition. No part of this thesis has been submitted for any other degree. Patrick Thomas Walsh December 2007 Acknowledgements Acknowledgements First, and foremost, I would like to thank, equally but for very different reasons, both of my supervisors, Professors Roger Downie and Pat Monaghan. I would like to thank Roger for his ‘open-door’ policy that has allowed many frank and helpful discussions about the contents of this thesis. Additionally, for allowing me the opportunity to greatly expand my CV by being given the option to help with the supervision of honours’ students and as much demonstrating as I could find time for. I would like to thank Pat for a great deal of patience and attention to detail, providing a perfect contrast to my frantic writing style and usually questionable grammatical knowledge. The excellent feedback received by her throughout my PhD has taught me to think more critically and write more concisely. I also need to thank Pat for the open invitation to her house to sample her pond for tadpoles throughout the year, and for often delivering the tadpoles directly to the department. I am hugely indebted to the Carnegie Trust for the Universities of Scotland for providing me with the funding for this studentship and the two small project grants, Universities UK for covering the extortionate over-seas fees, and the Glasgow Natural History Society for grants to purchase equipment. -

Histology of the Hepatopancreas and Anterior Intestine in the Freshwater Prawnmacrobrachium Carcinus
Nauplius ORIGINAL ARTICLE THE JOURNAL OF THE Histology of the hepatopancreas BRAZILIAN CRUSTACEAN SOCIETY and anterior intestine in the freshwater prawn Macrobrachium carcinus e-ISSN 2358-2936 www.scielo.br/nau (Crustacea, Decapoda) www.crustacea.org.br Thalles Fernando Rocha Ruiz1 orcid.org/0000-0003-0544-8325 Mateus Rossetto Vidal2 orcid.org/0000-0002-1532-5452 Karina Ribeiro3 orcid.org/0000-0001-7992-9717 Carlos Alberto Vicentini1,4 orcid.org/0000-0002-6816-033X Irene Bastos Franceschini Vicentini1,4 orcid.org/0000-0002-3877-7152 1 Biological Sciences Department, School of Sciences, São Paulo State University (Unesp). Campus Bauru, Brazil. TFRR E-mail: [email protected] CAV E-mail: [email protected] IBFV E-mail: [email protected] 2 Morphology Department, Institute of Biosciences, São Paulo State University (Unesp). Campus Botucatu, Brazil. MRV E-mail: [email protected] 3 Technology Center, Agricultural College of Jundiaí, Federal University of Rio Grande do Norte. RN, Brazil. KR E-mail: [email protected] 4 Aquaculture Center of São Paulo State University (Unesp). Brazil. ZOOBANK: http://zoobank.org/urn:lsid:zoobank.org:pub:3278494D-7D55-4A27- 8488-1837BC377C98 ABSTRACT The purpose of this study was to describe the structure of the midgut (hepatopancreas and intestine) in the endemic species, Macrobrachium carcinus. Thirty specimens were collected, and the midgut was fixed in Bouin’s solution for histological and histochemical analyzes by light microscopy. The hepatopancreas consists of two lobes that connect to the end of the stomach by primary ducts, which originate secondary tubules or hepatopancreatic ducts, that branch into hepatopancreatic tubules. -

Invertebrate Diversity in St Thomas Guts
DIVERSITY OF FRESHWATER FISH AND CRUSTACEANS OF ST. THOMAS WATERSHEDS AND ITS RELATIONSHIP TO WATER QUALITY AS AFFECTED BY RESIDENTIAL AND COMMERCIAL DEVELOPMENT WRRI Project 2006VI73B Prepared by: Donna Nemeth Renata Platenberg July 2007 Nemeth & Platenberg (2007) Diversity of freshwater fauna in St. Thomas gut streams DISCLAIMER The research on which this report is based was financed in part by the U. S. Department of the Interior, United States Geological Survey, through the Virgin Islands Water Resources Research Institute. The contents of this publication do not necessarily reflect the views and policies of the U. S. Department of the Interior, nor does mention of trade names or commercial products constitute their endorsement by the United States Government. ii Nemeth & Platenberg (2007) Diversity of freshwater fauna in St. Thomas gut streams ABSTRACT In the US Virgin Islands there has been considerable effort in surveying and mapping watersheds and riparian corridors. However, there has been little previous effort to document the freshwater systems, namely, the stormwater drainage guts. These guts form a vital connection between terrestrial habitats and upland activities and the downstream marine environment—yet research on the problems of non-point source pollution has largely overlooked the watershed habitat through which these pollutants are transported. Upland activities affect the levels of contaminants that flow through these habitats. We conducted a study to assess the impacts of levels of watershed development on the diversity of freshwater fauna. Three guts were selected that varied in development impact: Neltjeberg (low impact), Dorothea (moderate impact), and Turpentine Run (high impact). Freshwater habitats in a highly developed watershed contained more non-native fish species (guppies and tilapia) compared to those with low to moderate levels of development.