Product Innovation and Differentiation, Intra-Industry Trade and Growth
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Infant Antibiotic Exposure Search EMBASE 1. Exp Antibiotic Agent/ 2
Infant Antibiotic Exposure Search EMBASE 1. exp antibiotic agent/ 2. (Acedapsone or Alamethicin or Amdinocillin or Amdinocillin Pivoxil or Amikacin or Aminosalicylic Acid or Amoxicillin or Amoxicillin-Potassium Clavulanate Combination or Amphotericin B or Ampicillin or Anisomycin or Antimycin A or Arsphenamine or Aurodox or Azithromycin or Azlocillin or Aztreonam or Bacitracin or Bacteriocins or Bambermycins or beta-Lactams or Bongkrekic Acid or Brefeldin A or Butirosin Sulfate or Calcimycin or Candicidin or Capreomycin or Carbenicillin or Carfecillin or Cefaclor or Cefadroxil or Cefamandole or Cefatrizine or Cefazolin or Cefixime or Cefmenoxime or Cefmetazole or Cefonicid or Cefoperazone or Cefotaxime or Cefotetan or Cefotiam or Cefoxitin or Cefsulodin or Ceftazidime or Ceftizoxime or Ceftriaxone or Cefuroxime or Cephacetrile or Cephalexin or Cephaloglycin or Cephaloridine or Cephalosporins or Cephalothin or Cephamycins or Cephapirin or Cephradine or Chloramphenicol or Chlortetracycline or Ciprofloxacin or Citrinin or Clarithromycin or Clavulanic Acid or Clavulanic Acids or clindamycin or Clofazimine or Cloxacillin or Colistin or Cyclacillin or Cycloserine or Dactinomycin or Dapsone or Daptomycin or Demeclocycline or Diarylquinolines or Dibekacin or Dicloxacillin or Dihydrostreptomycin Sulfate or Diketopiperazines or Distamycins or Doxycycline or Echinomycin or Edeine or Enoxacin or Enviomycin or Erythromycin or Erythromycin Estolate or Erythromycin Ethylsuccinate or Ethambutol or Ethionamide or Filipin or Floxacillin or Fluoroquinolones -
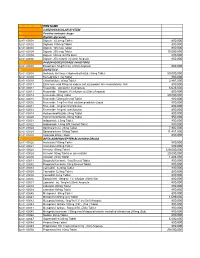
National Code Item Name 1
NATIONAL CODE ITEM NAME 1 CARDIOVASCULAR SYSTEM 1A Positive inotropic drugs 1AA Digtalis glycoside 02-01-00001 Digoxin 62.5mcg Tablet 800,000 02-01-00002 Digitoxin 100mcg Tablet 800,000 02-01-00003 Digoxin 125 mcg Tablet 800,000 02-01-00004 Digoxin 250 mcg Tablet 15,000,000 02-01-00005 Digoxin 50mcg /ml PG Elixir 800,000 02-01-00006 Digoxin 250 mcg/ml inj (2ml) Ampoule 800,000 1AB PHOSPHODIESTERASE INHIBITORS 02-01-00007 Enoximone 5mg/1ml inj (20ml) Ampoule 800,000 1B DIURETICS 02-01-00008 Amiloride Hcl 5mg + Hydrochlorthiazide 50mg Tablet 50,000,000 02-01-00009 Bumetanide 1 mg Tablet 800,000 02-01-00010 Chlorthalidone 50mg Tablet 2,867,000 02-01-00011 Ethacrynic acid 50mg as sodium salt inj (powder for reconstitution) Vial 800,000 02-01-00012 Frusemide 20mg/2ml inj Ampoule 6,625,000 02-01-00013 Frusemide 10mg/ml,I.V.infusion inj (25ml) Ampoule 800,000 02-01-00014 Frusemide 40mg Tablet 20,000,000 02-01-00015 Frusemide 500mg Scored Tablet 800,000 02-01-00016 Frusemide 1mg/1ml Oral solution peadiatric Liquid 800,000 02-01-00017 Frusemide 4mg/ml Oral Solution 800,000 02-01-00018 Frusemide 8mg/ml oral Solution 800,000 02-01-00019 Hydrochlorothiazide 25mg Tablet 800,000 02-01-00020 Hydrochlorothiazide 50mg Tablet 950,000 02-01-00021 Indapamide 2.5mg Tablet 800,000 02-01-00022 Indapamide 1.5mg S/R Coated Tablet 800,000 02-01-00023 Spironolactone 25mg Tablet 7,902,000 02-01-00024 Spironolactone 100mg Tablet 11,451,000 02-01-00025 Xipamide 20mg Tablet 800,000 1C BETA-ADRENOCEPTER BLOCKING DRUGS 02-01-00026 Acebutolol 100mg Tablet 800,000 02-01-00027 Acebutolol 200mg Tablet 800,000 02-01-00028 Atenolol 100mg Tablet 120,000,000 02-01-00029 Atenolol 50mg Tablet or (scored tab) 20,000,000 02-01-00030 Atenolol 25mg Tablet 1,483,000 02-01-00031 Bisoprolol fumarate 5mg Scored Tablet 800,000 02-01-00032 Bisoprolol fumarate 10mg Scored Tablet 800,000 02-01-00033 Carvedilol 6.25mg Tablet 800,000 02-01-00034 Carvedilol 12.5mg Tablet 800,000 02-01-00035 Carvedilol 25mg Tablet 800,000 02-01-00036 Esmolol Hcl 10mg/ml I.V. -

Partial Agreement in the Social and Public Health Field
COUNCIL OF EUROPE COMMITTEE OF MINISTERS (PARTIAL AGREEMENT IN THE SOCIAL AND PUBLIC HEALTH FIELD) RESOLUTION AP (88) 2 ON THE CLASSIFICATION OF MEDICINES WHICH ARE OBTAINABLE ONLY ON MEDICAL PRESCRIPTION (Adopted by the Committee of Ministers on 22 September 1988 at the 419th meeting of the Ministers' Deputies, and superseding Resolution AP (82) 2) AND APPENDIX I Alphabetical list of medicines adopted by the Public Health Committee (Partial Agreement) updated to 1 July 1988 APPENDIX II Pharmaco-therapeutic classification of medicines appearing in the alphabetical list in Appendix I updated to 1 July 1988 RESOLUTION AP (88) 2 ON THE CLASSIFICATION OF MEDICINES WHICH ARE OBTAINABLE ONLY ON MEDICAL PRESCRIPTION (superseding Resolution AP (82) 2) (Adopted by the Committee of Ministers on 22 September 1988 at the 419th meeting of the Ministers' Deputies) The Representatives on the Committee of Ministers of Belgium, France, the Federal Republic of Germany, Italy, Luxembourg, the Netherlands and the United Kingdom of Great Britain and Northern Ireland, these states being parties to the Partial Agreement in the social and public health field, and the Representatives of Austria, Denmark, Ireland, Spain and Switzerland, states which have participated in the public health activities carried out within the above-mentioned Partial Agreement since 1 October 1974, 2 April 1968, 23 September 1969, 21 April 1988 and 5 May 1964, respectively, Considering that the aim of the Council of Europe is to achieve greater unity between its members and that this -

Pharmaceuticals Appendix
)&f1y3X PHARMACEUTICAL APPENDIX TO THE HARMONIZED TARIFF SCHEDULE )&f1y3X PHARMACEUTICAL APPENDIX TO THE TARIFF SCHEDULE 3 Table 1. This table enumerates products described by International Non-proprietary Names (INN) which shall be entered free of duty under general note 13 to the tariff schedule. The Chemical Abstracts Service (CAS) registry numbers also set forth in this table are included to assist in the identification of the products concerned. For purposes of the tariff schedule, any references to a product enumerated in this table includes such product by whatever name known. Product CAS No. Product CAS No. ABAMECTIN 65195-55-3 ADAPALENE 106685-40-9 ABANOQUIL 90402-40-7 ADAPROLOL 101479-70-3 ABECARNIL 111841-85-1 ADEMETIONINE 17176-17-9 ABLUKAST 96566-25-5 ADENOSINE PHOSPHATE 61-19-8 ABUNIDAZOLE 91017-58-2 ADIBENDAN 100510-33-6 ACADESINE 2627-69-2 ADICILLIN 525-94-0 ACAMPROSATE 77337-76-9 ADIMOLOL 78459-19-5 ACAPRAZINE 55485-20-6 ADINAZOLAM 37115-32-5 ACARBOSE 56180-94-0 ADIPHENINE 64-95-9 ACEBROCHOL 514-50-1 ADIPIODONE 606-17-7 ACEBURIC ACID 26976-72-7 ADITEREN 56066-19-4 ACEBUTOLOL 37517-30-9 ADITOPRIME 56066-63-8 ACECAINIDE 32795-44-1 ADOSOPINE 88124-26-9 ACECARBROMAL 77-66-7 ADOZELESIN 110314-48-2 ACECLIDINE 827-61-2 ADRAFINIL 63547-13-7 ACECLOFENAC 89796-99-6 ADRENALONE 99-45-6 ACEDAPSONE 77-46-3 AFALANINE 2901-75-9 ACEDIASULFONE SODIUM 127-60-6 AFLOQUALONE 56287-74-2 ACEDOBEN 556-08-1 AFUROLOL 65776-67-2 ACEFLURANOL 80595-73-9 AGANODINE 86696-87-9 ACEFURTIAMINE 10072-48-7 AKLOMIDE 3011-89-0 ACEFYLLINE CLOFIBROL 70788-27-1 -

E3 Appendix 1 (Part 1 of 2): Search Strategy Used in MEDLINE
This single copy is for your personal, non-commercial use only. For permission to reprint multiple copies or to order presentation-ready copies for distribution, contact CJHP at [email protected] Appendix 1 (part 1 of 2): Search strategy used in MEDLINE # Searches 1 exp *anti-bacterial agents/ or (antimicrobial* or antibacterial* or antibiotic* or antiinfective* or anti-microbial* or anti-bacterial* or anti-biotic* or anti- infective* or “ß-lactam*” or b-Lactam* or beta-Lactam* or ampicillin* or carbapenem* or cephalosporin* or clindamycin or erythromycin or fluconazole* or methicillin or multidrug or multi-drug or penicillin* or tetracycline* or vancomycin).kf,kw,ti. or (antimicrobial or antibacterial or antiinfective or anti-microbial or anti-bacterial or anti-infective or “ß-lactam*” or b-Lactam* or beta-Lactam* or ampicillin* or carbapenem* or cephalosporin* or c lindamycin or erythromycin or fluconazole* or methicillin or multidrug or multi-drug or penicillin* or tetracycline* or vancomycin).ab. /freq=2 2 alamethicin/ or amdinocillin/ or amdinocillin pivoxil/ or amikacin/ or amoxicillin/ or amphotericin b/ or ampicillin/ or anisomycin/ or antimycin a/ or aurodox/ or azithromycin/ or azlocillin/ or aztreonam/ or bacitracin/ or bacteriocins/ or bambermycins/ or bongkrekic acid/ or brefeldin a/ or butirosin sulfate/ or calcimycin/ or candicidin/ or capreomycin/ or carbenicillin/ or carfecillin/ or cefaclor/ or cefadroxil/ or cefamandole/ or cefatrizine/ or cefazolin/ or cefixime/ or cefmenoxime/ or cefmetazole/ or cefonicid/ or cefoperazone/ -

F1y3x CHAPTER 29 ORGANIC CHEMICALS VI 29-1 Notes 1
)&f1y3X CHAPTER 29 ORGANIC CHEMICALS VI 29-1 Notes 1. Except where the context otherwise requires, the headings of this chapter apply only to: (a) Separate chemically defined organic compounds, whether or not containing impurities; (b) Mixtures of two or more isomers of the same organic compound (whether or not containing impurities), except mixtures of acyclic hydrocarbon isomers (other than stereoisomers), whether or not saturated (chapter 27); (c) The products of headings 2936 to 2939 or the sugar ethers and sugar esters, and their salts, of heading 2940, or the products of heading 2941, whether or not chemically defined; (d) Products mentioned in (a), (b) or (c) above dissolved in water; (e) Products mentioned in (a), (b) or (c) above dissolved in other solvents provided that the solution constitutes a normal and necessary method of putting up these products adopted solely for reasons of safety or for transport and that the solvent does not render the product particularly suitable for specific use rather than for general use; (f) The products mentioned in (a), (b), (c), (d) or (e) above with an added stabilizer necessary for their preservation or transport; (g) The products mentioned in (a), (b), (c), (d), (e) or (f) above with an added antidusting agent or a coloring or odoriferous substance added to facilitate their identification or for safety reasons, provided that the additions do not render the product particularly suitable for specific use rather than for general use; (h) The following products, diluted to standard strengths, for the production of azo dyes: diazonium salts, couplers used for these salts and diazotizable amines and their salts. -

Federal Register / Vol. 60, No. 80 / Wednesday, April 26, 1995 / Notices DIX to the HTSUS—Continued
20558 Federal Register / Vol. 60, No. 80 / Wednesday, April 26, 1995 / Notices DEPARMENT OF THE TREASURY Services, U.S. Customs Service, 1301 TABLE 1.ÐPHARMACEUTICAL APPEN- Constitution Avenue NW, Washington, DIX TO THE HTSUSÐContinued Customs Service D.C. 20229 at (202) 927±1060. CAS No. Pharmaceutical [T.D. 95±33] Dated: April 14, 1995. 52±78±8 ..................... NORETHANDROLONE. A. W. Tennant, 52±86±8 ..................... HALOPERIDOL. Pharmaceutical Tables 1 and 3 of the Director, Office of Laboratories and Scientific 52±88±0 ..................... ATROPINE METHONITRATE. HTSUS 52±90±4 ..................... CYSTEINE. Services. 53±03±2 ..................... PREDNISONE. 53±06±5 ..................... CORTISONE. AGENCY: Customs Service, Department TABLE 1.ÐPHARMACEUTICAL 53±10±1 ..................... HYDROXYDIONE SODIUM SUCCI- of the Treasury. NATE. APPENDIX TO THE HTSUS 53±16±7 ..................... ESTRONE. ACTION: Listing of the products found in 53±18±9 ..................... BIETASERPINE. Table 1 and Table 3 of the CAS No. Pharmaceutical 53±19±0 ..................... MITOTANE. 53±31±6 ..................... MEDIBAZINE. Pharmaceutical Appendix to the N/A ............................. ACTAGARDIN. 53±33±8 ..................... PARAMETHASONE. Harmonized Tariff Schedule of the N/A ............................. ARDACIN. 53±34±9 ..................... FLUPREDNISOLONE. N/A ............................. BICIROMAB. 53±39±4 ..................... OXANDROLONE. United States of America in Chemical N/A ............................. CELUCLORAL. 53±43±0 -

(12) United States Patent (10) Patent No.: US 7,504,381 B2 Mor Et Al
USOO7504381 B2 (12) United States Patent (10) Patent No.: US 7,504,381 B2 Mor et al. (45) Date of Patent: Mar. 17, 2009 (54) ANTIMICROBIAL AGENTS Tossi et al. "Amphipathic, Alpha-Helical Antimicrobial Peptides'. Biopolymers, 55(1): 4-30, 2000. Abstract, Table 1, p. 7-8, Table II, p. (75) Inventors: Amram Mor, Haifa (IL); Inna 13. Radzishevsky, Haifa (IL) Huang "Peptide-Lipid Interactions and Mechanisms of Antimicro bial Peptides”, Novartis Found Symposium, 225: 188-200, 1999. (73) Assignee: Technion Research & Development Discussion 200-206. Epand et al. “Mechanisms for the Modulation of Membrane Bilayer Foundation Ltd., Haifa (IL) Properties by Amphipathic Helical Peptides'. Biopolymers, 37(5): 319-338, 1995. Abstract. (*) Notice: Subject to any disclaimer, the term of this Ono et al. “Design and Synthesis of Basic Peptides Having patent is extended or adjusted under 35 Amphipathic Beta-Structure and Their Interaction With U.S.C. 154(b) by 153 days. Phospholipid Membranes'. Biochimica et Biophysica Acta (BBA) Biomembranes, 1022(2): 237-244, 1990. Abstract. (21) Appl. No.: 11/234,183 Aarbiou et al. “Human Neutrophil Defensins Induce Lung Epithelial Cell Proliferation. In Vitro”, Journal of Leukocyte Biology, 72: 167 (22) Filed: Sep. 26, 2005 174, 2002. Acar “Consequences of Bacterial Resistance to Antibiotics in Medi (65) Prior Publication Data cal Practice'. Clinical Infectious Diseases, 24(Suppl. 1): S17-S18. 1997. US 2006/OO74O21 A1 Apr. 6, 2006 Alan et al. “Expression of A Magainin-Type Antimicrobial Peptide Gene (MSI-99) in Tomato Enhances Resistance to Bacterial Speck Related U.S. Application Data Disease'. Plant Cell Reports, 22:388-396, 2004. -

(12) United States Patent (10) Patent No.: US 8,383,154 B2 Bar-Shalom Et Al
USOO8383154B2 (12) United States Patent (10) Patent No.: US 8,383,154 B2 Bar-Shalom et al. (45) Date of Patent: Feb. 26, 2013 (54) SWELLABLE DOSAGE FORM COMPRISING W W 2.3. A. 3. 2. GELLAN GUMI WO WOO1,76610 10, 2001 WO WOO2,46571 A2 6, 2002 (75) Inventors: Daniel Bar-Shalom, Kokkedal (DK); WO WO O2/49571 A2 6, 2002 Lillian Slot, Virum (DK); Gina Fischer, WO WO 03/043638 A1 5, 2003 yerlosea (DK), Pernille Heyrup WO WO 2004/096906 A1 11, 2004 Hemmingsen, Bagsvaerd (DK) WO WO 2005/007074 1, 2005 WO WO 2005/007074 A 1, 2005 (73) Assignee: Egalet A/S, Vaerlose (DK) OTHER PUBLICATIONS (*) Notice: Subject to any disclaimer, the term of this patent is extended or adjusted under 35 JECFA, “Gellangum”. FNP 52 Addendum 4 (1996).* U.S.C. 154(b) by 1259 days. JECFA, “Talc”, FNP 52 Addendum 1 (1992).* Alterna LLC, “ElixSure, Allergy Formula', description and label (21) Appl. No.: 111596,123 directions, online (Feb. 6, 2007). Hagerström, H., “Polymer gels as pharmaceutical dosage forms'. (22) PCT Filed: May 11, 2005 comprehensive Summaries of Uppsala dissertations from the faculty of pharmacy, vol. 293 Uppsala (2003). (86). PCT No.: PCT/DK2OOS/OOO317 Lin, “Gellan Gum', U.S. Food and Drug Administration, www. inchem.org, online (Jan. 17, 2005). S371 (c)(1), Miyazaki, S., et al., “In situ-gelling gellan formulations as vehicles (2), (4) Date: Aug. 14, 2007 for oral drug delivery”. J. Control Release, vol. 60, pp. 287-295 (1999). (87) PCT Pub. No.: WO2005/107713 Rowe, Raymond C. -

Visão De Futuro Para Produção De Antibióticos: Tendências De Pesquisa, Desenvolvimento E Inovação
UNIVERSIDADE FEDERAL DO RIO DE JANEIRO CRISTINA D’URSO DE SOUZA MENDES SANTOS VISÃO DE FUTURO PARA PRODUÇÃO DE ANTIBIÓTICOS: TENDÊNCIAS DE PESQUISA, DESENVOLVIMENTO E INOVAÇÃO Rio de Janeiro EQ/UFRJ 2014 CRISTINA D ’U RSO DE SOUZA MENDES SANTOS VISÃO DE FUTURO PARA A PRODUÇÃO DE ANTIBIÓTICOS: TENDÊNCIAS DE PESQUISA, DESENVOLVIMENTO E INOVAÇÃO Tese de Doutorado apresentada ao Programa de Pós-Graduação em Tecnologia de Processos Químicos e Bioquímicos, Escola de Química, Universidade Federal do Rio de Janeiro, como requisito parcial à obtenção do título de Doutor em Ciências, D.Sc. Orientadora: Profa. Adelaide Maria de Souza Antunes, D.Sc. Rio de Janeiro 2014 Santos, Cristina d’Urso de Souza Mendes. Visão de futuro para produção de antibióticos: tendências de pesquisa, desenvolvimento e inovação / Cristina d’Urso de Souza Mendes Santos. - Rio de Janeiro, 2014. 216 f.: il.; 29,7 cm. Tese (Doutorado em Ciências) – Universidade Federal do Rio de Janeiro, Escola de Química, Programa de Pós-Graduação em Tecnologia de Processos Químicos e Bioquímicos, Rio de Janeiro, 2014. Orientadora: Adelaide Maria de Souza Antunes. 1. Antibióticos. 2. P&D na Indústria Farmacêutica. 3. Prospecção Tecnológica. 4. Patentes. I. Antunes, Adelaide Maria de Souza. II. Universidade Federal do Rio de Janeiro. Escola de Química. III. Visão de futuro para a produção de antibióticos: tendências de pesquisa, desenvolvimento e inovação. iv v Dedico esta tese à minha mãe querida e amada, que está no céu comemorando esta vitória, que é mais dela do que minha. Dedico também à minha filhinha Malu que sem entender foi a minha maior motivação para concluir esta tese. -

Stembook 2018.Pdf
The use of stems in the selection of International Nonproprietary Names (INN) for pharmaceutical substances FORMER DOCUMENT NUMBER: WHO/PHARM S/NOM 15 WHO/EMP/RHT/TSN/2018.1 © World Health Organization 2018 Some rights reserved. This work is available under the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 IGO licence (CC BY-NC-SA 3.0 IGO; https://creativecommons.org/licenses/by-nc-sa/3.0/igo). Under the terms of this licence, you may copy, redistribute and adapt the work for non-commercial purposes, provided the work is appropriately cited, as indicated below. In any use of this work, there should be no suggestion that WHO endorses any specific organization, products or services. The use of the WHO logo is not permitted. If you adapt the work, then you must license your work under the same or equivalent Creative Commons licence. If you create a translation of this work, you should add the following disclaimer along with the suggested citation: “This translation was not created by the World Health Organization (WHO). WHO is not responsible for the content or accuracy of this translation. The original English edition shall be the binding and authentic edition”. Any mediation relating to disputes arising under the licence shall be conducted in accordance with the mediation rules of the World Intellectual Property Organization. Suggested citation. The use of stems in the selection of International Nonproprietary Names (INN) for pharmaceutical substances. Geneva: World Health Organization; 2018 (WHO/EMP/RHT/TSN/2018.1). Licence: CC BY-NC-SA 3.0 IGO. Cataloguing-in-Publication (CIP) data. -

ALPHABETICAL INDEX Harmonized Tariff Schedule of the United States (2010) (Rev
Harmonized Tariff Schedule of the United States (2010) (Rev. 2) Annotated for Statistical Reporting Purposes ALPHABETICAL INDEX Harmonized Tariff Schedule of the United States (2010) (Rev. 2) Annotated for Statistical Reporting Purposes ALPHABETICAL INDEX ABACA FIBERS ....................................5305.21-29 ACRYLONITRILE-BUTADIENE RUBBER (NBR) ABRASIVE POWDER uncompounded .................................. 4002.51-59 on a base of textile material, paper, paperboard or other ACRYLONITRILE-BUTADIENE-STYRENE (ABS) COPOLYMERS materials ........................................6805.10-30 in primary forms ....................................3903.30 ABRASIVE WHEELS.................................. 6804.22 ADDITIVES ABRASIVES prepared, for cements, mortars or concretes ..............3823.40 natural..........................................2513.21-29 prepared, for mineral oils .......................... 3811.11-90 ABSOLUTES ADDRESS BOOKS essential oil...........................................3301 of paper or paperboard ...............................4820.10 AC GENERATORS ...................... 8501.61-64, 8502.11-30 ADDRESS PLATE EMBOSSING MACHINES ...............8472.20 AC MOTORS........................... 8501.10-20, 8501.40-53 ADDRESS PLATES ACAJOU D'AFRIQUE .................................. Ch. 44 of base metals......................................8310.00 ACCELERATORS ADDRESSING MACHINES ..............................8472.20 chemical reaction ......................................3815 ADHESIVE PAPER AND PAPERBOARD . 4811.21-29, 4821,