Identification, Cloning and Expressions of Proteases from a Cold Adapted Organism Aliivibrio Salmonicida
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

(12) United States Patent (10) Patent No.: US 6,395,889 B1 Robison (45) Date of Patent: May 28, 2002
USOO6395889B1 (12) United States Patent (10) Patent No.: US 6,395,889 B1 Robison (45) Date of Patent: May 28, 2002 (54) NUCLEIC ACID MOLECULES ENCODING WO WO-98/56804 A1 * 12/1998 ........... CO7H/21/02 HUMAN PROTEASE HOMOLOGS WO WO-99/0785.0 A1 * 2/1999 ... C12N/15/12 WO WO-99/37660 A1 * 7/1999 ........... CO7H/21/04 (75) Inventor: fish E. Robison, Wilmington, MA OTHER PUBLICATIONS Vazquez, F., et al., 1999, “METH-1, a human ortholog of (73) Assignee: Millennium Pharmaceuticals, Inc., ADAMTS-1, and METH-2 are members of a new family of Cambridge, MA (US) proteins with angio-inhibitory activity', The Journal of c: - 0 Biological Chemistry, vol. 274, No. 33, pp. 23349–23357.* (*) Notice: Subject to any disclaimer, the term of this Descriptors of Protease Classes in Prosite and Pfam Data patent is extended or adjusted under 35 bases. U.S.C. 154(b) by 0 days. * cited by examiner (21) Appl. No.: 09/392, 184 Primary Examiner Ponnathapu Achutamurthy (22) Filed: Sep. 9, 1999 ASSistant Examiner William W. Moore (51) Int. Cl." C12N 15/57; C12N 15/12; (74) Attorney, Agent, or Firm-Alston & Bird LLP C12N 9/64; C12N 15/79 (57) ABSTRACT (52) U.S. Cl. .................... 536/23.2; 536/23.5; 435/69.1; 435/252.3; 435/320.1 The invention relates to polynucleotides encoding newly (58) Field of Search ............................... 536,232,235. identified protease homologs. The invention also relates to 435/6, 226, 69.1, 252.3 the proteases. The invention further relates to methods using s s s/ - - -us the protease polypeptides and polynucleotides as a target for (56) References Cited diagnosis and treatment in protease-mediated disorders. -

Structural and Biochemical Characterizations of Three Potential Drug Targets from Pathogens
Digital Comprehensive Summaries of Uppsala Dissertations from the Faculty of Science and Technology 2020 Structural and Biochemical Characterizations of Three Potential Drug Targets from Pathogens LU LU ACTA UNIVERSITATIS UPSALIENSIS ISSN 1651-6214 ISBN 978-91-513-1148-7 UPPSALA urn:nbn:se:uu:diva-435815 2021 Dissertation presented at Uppsala University to be publicly examined in Room A1:111a, BMC, Husargatan 3, Uppsala, Friday, 16 April 2021 at 13:15 for the degree of Doctor of Philosophy. The examination will be conducted in English. Faculty examiner: Christian Cambillau. Abstract Lu, L. 2021. Structural and Biochemical Characterizations of Three Potential Drug Targets from Pathogens. Digital Comprehensive Summaries of Uppsala Dissertations from the Faculty of Science and Technology 2020. 91 pp. Uppsala: Acta Universitatis Upsaliensis. ISBN 978-91-513-1148-7. As antibiotic resistance of various pathogens emerged globally, the need for new effective drugs with novel modes of action became urgent. In this thesis, we focus on infectious diseases, e.g. tuberculosis, malaria, and nosocomial infections, and the corresponding causative pathogens, Mycobacterium tuberculosis, Plasmodium falciparum, and the Gram-negative ESKAPE pathogens that underlie so many healthcare-acquired diseases. Following the same- target-other-pathogen (STOP) strategy, we attempted to comprehensively explore the properties of three promising drug targets. Signal peptidase I (SPase I), existing both in Gram-negative and Gram-positive bacteria, as well as in parasites, is vital for cell viability, due to its critical role in signal peptide cleavage, thus, protein maturation, and secreted protein transport. Three factors, comprising essentiality, a unique mode of action, and easy accessibility, make it an attractive drug target. -

B1–Proteases As Molecular Targets of Drug Development
Abstracts B1–Proteases as Molecular Targets of Drug Development B1-001 lin release from the beta cells. Furthermore, GLP-1 also stimu- DPP-IV structure and inhibitor design lates beta cell growth and insulin biosynthesis, inhibits glucagon H. B. Rasmussen1, S. Branner1, N. Wagtmann3, J. R. Bjelke1 and secretion, reduces free fatty acids and delays gastric emptying. A. B. Kanstrup2 GLP-1 has therefore been suggested as a potentially new treat- 1Protein Engineering, Novo Nordisk A/S, Bagsvaerd, Denmark, ment for type 2 diabetes. However, GLP-1 is very rapidly degra- 2Medicinal Chemistry, Novo Nordisk A/S, Maaloev, Denmark, ded in the bloodstream by the enzyme dipeptidyl peptidase IV 3Discovery Biology, Novo Nordisk A/S, Maaloev, DENMARK. (DPP-IV; EC 3.4.14.5). A very promising approach to harvest E-mail: [email protected] the beneficial effect of GLP-1 in the treatment of diabetes is to inhibit the DPP-IV enzyme, thereby enhancing the levels of The incretin hormones GLP-1 and GIP are released from the gut endogenously intact circulating GLP-1. The three dimensional during meals, and serve as enhancers of glucose stimulated insu- structure of human DPP-IV in complex with various inhibitors 138 Abstracts creates a better understanding of the specificity and selectivity of drug-like transition-state inhibitors but can be utilized for the this enzyme and allows for further exploration and design of new design of non-transition-state inhibitors that compete for sub- therapeutic inhibitors. The majority of the currently known DPP- strate binding. Besides carrying out proteolytic activity, the IV inhibitors consist of an alpha amino acid pyrrolidine core, to ectodomain of memapsin 2 also interacts with APP leading to which substituents have been added to optimize affinity, potency, the endocytosis of both proteins into the endosomes where APP enzyme selectivity, oral bioavailability, and duration of action. -

Infant Antibiotic Exposure Search EMBASE 1. Exp Antibiotic Agent/ 2
Infant Antibiotic Exposure Search EMBASE 1. exp antibiotic agent/ 2. (Acedapsone or Alamethicin or Amdinocillin or Amdinocillin Pivoxil or Amikacin or Aminosalicylic Acid or Amoxicillin or Amoxicillin-Potassium Clavulanate Combination or Amphotericin B or Ampicillin or Anisomycin or Antimycin A or Arsphenamine or Aurodox or Azithromycin or Azlocillin or Aztreonam or Bacitracin or Bacteriocins or Bambermycins or beta-Lactams or Bongkrekic Acid or Brefeldin A or Butirosin Sulfate or Calcimycin or Candicidin or Capreomycin or Carbenicillin or Carfecillin or Cefaclor or Cefadroxil or Cefamandole or Cefatrizine or Cefazolin or Cefixime or Cefmenoxime or Cefmetazole or Cefonicid or Cefoperazone or Cefotaxime or Cefotetan or Cefotiam or Cefoxitin or Cefsulodin or Ceftazidime or Ceftizoxime or Ceftriaxone or Cefuroxime or Cephacetrile or Cephalexin or Cephaloglycin or Cephaloridine or Cephalosporins or Cephalothin or Cephamycins or Cephapirin or Cephradine or Chloramphenicol or Chlortetracycline or Ciprofloxacin or Citrinin or Clarithromycin or Clavulanic Acid or Clavulanic Acids or clindamycin or Clofazimine or Cloxacillin or Colistin or Cyclacillin or Cycloserine or Dactinomycin or Dapsone or Daptomycin or Demeclocycline or Diarylquinolines or Dibekacin or Dicloxacillin or Dihydrostreptomycin Sulfate or Diketopiperazines or Distamycins or Doxycycline or Echinomycin or Edeine or Enoxacin or Enviomycin or Erythromycin or Erythromycin Estolate or Erythromycin Ethylsuccinate or Ethambutol or Ethionamide or Filipin or Floxacillin or Fluoroquinolones -

(12) United States Patent (10) Patent No.: US 8,603,824 B2 Ramseier Et Al
USOO8603824B2 (12) United States Patent (10) Patent No.: US 8,603,824 B2 Ramseier et al. (45) Date of Patent: Dec. 10, 2013 (54) PROCESS FOR IMPROVED PROTEIN 5,399,684 A 3, 1995 Davie et al. EXPRESSION BY STRAIN ENGINEERING 5,418, 155 A 5/1995 Cormier et al. 5,441,934 A 8/1995 Krapcho et al. (75) Inventors: Thomas M. Ramseier, Poway, CA 5,508,192 A * 4/1996 Georgiou et al. .......... 435/252.3 (US); Hongfan Jin, San Diego, CA 5,527,883 A 6/1996 Thompson et al. (US); Charles H. Squires, Poway, CA 5,558,862 A 9, 1996 Corbinet al. 5,559,015 A 9/1996 Capage et al. (US) 5,571,694 A 11/1996 Makoff et al. (73) Assignee: Pfenex, Inc., San Diego, CA (US) 5,595,898 A 1/1997 Robinson et al. 5,610,044 A 3, 1997 Lam et al. (*) Notice: Subject to any disclaimer, the term of this 5,621,074 A 4/1997 Bjorn et al. patent is extended or adjusted under 35 5,622,846 A 4/1997 Kiener et al. 5,641,671 A 6/1997 Bos et al. U.S.C. 154(b) by 471 days. 5,641,870 A 6/1997 Rinderknecht et al. 5,643,774 A 7/1997 Ligon et al. (21) Appl. No.: 11/189,375 5,662,898 A 9/1997 Ligon et al. (22) Filed: Jul. 26, 2005 5,677,127 A 10/1997 Hogan et al. 5,683,888 A 1 1/1997 Campbell (65) Prior Publication Data 5,686,282 A 11/1997 Lam et al. -

Serine Proteases with Altered Sensitivity to Activity-Modulating
(19) & (11) EP 2 045 321 A2 (12) EUROPEAN PATENT APPLICATION (43) Date of publication: (51) Int Cl.: 08.04.2009 Bulletin 2009/15 C12N 9/00 (2006.01) C12N 15/00 (2006.01) C12Q 1/37 (2006.01) (21) Application number: 09150549.5 (22) Date of filing: 26.05.2006 (84) Designated Contracting States: • Haupts, Ulrich AT BE BG CH CY CZ DE DK EE ES FI FR GB GR 51519 Odenthal (DE) HU IE IS IT LI LT LU LV MC NL PL PT RO SE SI • Coco, Wayne SK TR 50737 Köln (DE) •Tebbe, Jan (30) Priority: 27.05.2005 EP 05104543 50733 Köln (DE) • Votsmeier, Christian (62) Document number(s) of the earlier application(s) in 50259 Pulheim (DE) accordance with Art. 76 EPC: • Scheidig, Andreas 06763303.2 / 1 883 696 50823 Köln (DE) (71) Applicant: Direvo Biotech AG (74) Representative: von Kreisler Selting Werner 50829 Köln (DE) Patentanwälte P.O. Box 10 22 41 (72) Inventors: 50462 Köln (DE) • Koltermann, André 82057 Icking (DE) Remarks: • Kettling, Ulrich This application was filed on 14-01-2009 as a 81477 München (DE) divisional application to the application mentioned under INID code 62. (54) Serine proteases with altered sensitivity to activity-modulating substances (57) The present invention provides variants of ser- screening of the library in the presence of one or several ine proteases of the S1 class with altered sensitivity to activity-modulating substances, selection of variants with one or more activity-modulating substances. A method altered sensitivity to one or several activity-modulating for the generation of such proteases is disclosed, com- substances and isolation of those polynucleotide se- prising the provision of a protease library encoding poly- quences that encode for the selected variants. -

Subtilase Variants
(19) & (11) EP 2 333 055 A1 (12) EUROPEAN PATENT APPLICATION (43) Date of publication: (51) Int Cl.: 15.06.2011 Bulletin 2011/24 C12N 9/54 (2006.01) C11D 3/386 (2006.01) C12N 1/15 (2006.01) C12N 1/19 (2006.01) (2006.01) (21) Application number: 10180093.6 C12N 1/21 (22) Date of filing: 12.10.2001 (84) Designated Contracting States: (72) Inventors: AT BE CH CY DE DK ES FI FR GB GR IE IT LI LU • Nørregaard-Madsen, Mads MC NL PT SE TR DK-3560, Birkerød (DK) • Larsen, Line, Bloch (30) Priority: 13.10.2000 DK 200001528 DK-2880, Bagsværd (DK) • Hansen, Peter Kamp (62) Document number(s) of the earlier application(s) in DK-4320, Lejre (DK) accordance with Art. 76 EPC: 01978206.9 / 1 326 966 Remarks: This application was filed on 27-09-2010 as a (71) Applicant: Novozymes A/S divisional application to the application mentioned 2880 Bagsvaerd (DK) under INID code 62. (54) Subtilase variants (57) Novel subtilase variants having a reduced ten- dry detergent compositions and dishwash composition, dency towards inhibition by substances present in eggs, including automatic dishwash compositions. Also, isolat- such as trypsin inhibitor type IV- 0. In particular, variants ed DNA sequences encoding the variants, expression comprising at least one additional amino acid residue vectors, host cells, and methods for producing and using between positions 42-43, 51-56, 155-161, 187-190, the variants of the invention. Further, cleaning and de- 216-217, 217-218 or 218-219 (in BASBPN numbering). tergent compositions comprising the variants are dis- These subtilase variants are useful exhibiting excellent closed. -
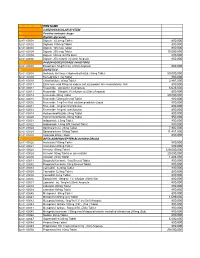
National Code Item Name 1
NATIONAL CODE ITEM NAME 1 CARDIOVASCULAR SYSTEM 1A Positive inotropic drugs 1AA Digtalis glycoside 02-01-00001 Digoxin 62.5mcg Tablet 800,000 02-01-00002 Digitoxin 100mcg Tablet 800,000 02-01-00003 Digoxin 125 mcg Tablet 800,000 02-01-00004 Digoxin 250 mcg Tablet 15,000,000 02-01-00005 Digoxin 50mcg /ml PG Elixir 800,000 02-01-00006 Digoxin 250 mcg/ml inj (2ml) Ampoule 800,000 1AB PHOSPHODIESTERASE INHIBITORS 02-01-00007 Enoximone 5mg/1ml inj (20ml) Ampoule 800,000 1B DIURETICS 02-01-00008 Amiloride Hcl 5mg + Hydrochlorthiazide 50mg Tablet 50,000,000 02-01-00009 Bumetanide 1 mg Tablet 800,000 02-01-00010 Chlorthalidone 50mg Tablet 2,867,000 02-01-00011 Ethacrynic acid 50mg as sodium salt inj (powder for reconstitution) Vial 800,000 02-01-00012 Frusemide 20mg/2ml inj Ampoule 6,625,000 02-01-00013 Frusemide 10mg/ml,I.V.infusion inj (25ml) Ampoule 800,000 02-01-00014 Frusemide 40mg Tablet 20,000,000 02-01-00015 Frusemide 500mg Scored Tablet 800,000 02-01-00016 Frusemide 1mg/1ml Oral solution peadiatric Liquid 800,000 02-01-00017 Frusemide 4mg/ml Oral Solution 800,000 02-01-00018 Frusemide 8mg/ml oral Solution 800,000 02-01-00019 Hydrochlorothiazide 25mg Tablet 800,000 02-01-00020 Hydrochlorothiazide 50mg Tablet 950,000 02-01-00021 Indapamide 2.5mg Tablet 800,000 02-01-00022 Indapamide 1.5mg S/R Coated Tablet 800,000 02-01-00023 Spironolactone 25mg Tablet 7,902,000 02-01-00024 Spironolactone 100mg Tablet 11,451,000 02-01-00025 Xipamide 20mg Tablet 800,000 1C BETA-ADRENOCEPTER BLOCKING DRUGS 02-01-00026 Acebutolol 100mg Tablet 800,000 02-01-00027 Acebutolol 200mg Tablet 800,000 02-01-00028 Atenolol 100mg Tablet 120,000,000 02-01-00029 Atenolol 50mg Tablet or (scored tab) 20,000,000 02-01-00030 Atenolol 25mg Tablet 1,483,000 02-01-00031 Bisoprolol fumarate 5mg Scored Tablet 800,000 02-01-00032 Bisoprolol fumarate 10mg Scored Tablet 800,000 02-01-00033 Carvedilol 6.25mg Tablet 800,000 02-01-00034 Carvedilol 12.5mg Tablet 800,000 02-01-00035 Carvedilol 25mg Tablet 800,000 02-01-00036 Esmolol Hcl 10mg/ml I.V. -

Diapause in the Mosquito Culex Pipiens Evokes a Metabolic Switch from Blood Feeding to Sugar Gluttony
Diapause in the mosquito Culex pipiens evokes a metabolic switch from blood feeding to sugar gluttony Rebecca M. Robich* and David L. Denlinger† Department of Entomology, Ohio State University, 318 West 12th Avenue, Columbus, OH 43210 Contributed by David L. Denlinger, September 12, 2005 A key characteristic of overwintering dormancy (diapause) in the Although the physiological and ecological aspects of blood and mosquito Culex pipiens is the switch in females from blood feeding sugar feeding in diapausing C. pipiens have been well described, the to sugar gluttony. We present evidence demonstrating that genes molecular events that contribute to this metabolic decision have not encoding enzymes needed to digest a blood meal (trypsin and a been explored. In this study, we used suppressive subtractive chymotrypsin-like protease) are down-regulated in diapause-des- hybridization (SSH) to isolate three clones linked to this metabolic tined females, and that concurrently, a gene associated with the decision: fatty acid synthase, trypsin, and chymotrypsin-like serine accumulation of lipid reserves (fatty acid synthase) is highly up- protease. We then used these clones to probe the metabolic path- regulated. As the females then enter diapause, fatty acid synthase ways associated with the mosquito’s metabolic decision to enter and is only sporadically expressed, and expression of trypsin and terminate diapause. Because both short day length and low tem- chymotrypsin-like remains undetectable. Late in diapause (2–3 perature program diapause in C. pipiens, we also distinguished months at 18°C), the genes encoding the digestive enzymes begin between temperature and photoperiodic effects. We concluded to be expressed as the female prepares to take a blood meal upon that the short-day programming of diapause results in the down- the termination of diapause. -

Wsn 40 (2016) 147-162 Eissn 2392-2192
Available online at www.worldscientificnews.com WSN 40 (2016) 147-162 EISSN 2392-2192 Utilization of mulberry leaves treated with seed powder cowpea, Vigna unguiculata (L) for feeding the fifth instar larvae of silkworm, Bombyx mori (L) (Race: PM x CSR2) Vitthalrao B. Khyade1,*, Atharv Atul Gosavi2 1Department of Zoology, Shardabai Pawar Mahila Mahavidyalaya, Shardanagar Tal. Baramati; Dist. Pune - 413115, India 2Agriculture Development Trust Agri Polytechnic, Sharadanagar, Malegaon Colony, Tal: Baramati, Dist: Pune. PIN: 413115 Maharashtra, India *E-mail address: [email protected] ABSTRACT The present attempt was to screen the changes in the cocoon parameters; silk filament parameters and activities of biochemical reactions catalyzed by the midgut enzymes fifth instsr larvae of silkworm fed with mulberry leaves treated with aqueous solution of seed powder of Cowpeas (Vigna unguiculata). The cowpea seed powder was dissolved in distilled water and diluted to 2.5%, 5%, 7.5%, and 10% concentrations. Fresh mulberry leaves were dipped in each concentration of aqueous solution of cowpea seed powder for half an hour. 1000 ml solution was used for 100 grams of mulberry leaves. Treated mulberry leaves were drained off completely and then used for feeding. The mulberry leaves were fed five times per day at the rate of 100 grams per 100 larvae for each time. Untreated group of larvae were feed with untreated mulberry leaves. Water treated group of larvae were feed with water treated mulberry leaves. The experimental groups of larvae were feed with feed separately with 2.5 percent cowpea treated; 5.00 percent cowpea treated; 7.5 percent cowpea treated and 10.00 percent cowpea treated mulberry leaves. -

Fibronectin-Binding Protein B (Fnbpb)
ARTICLE cro Fibronectin-binding protein B (FnBPB) from Staphylococcus aureus protects against the antimicrobial activity of histones Received for publication, September 5, 2018, and in revised form, December 17, 2018 Published, Papers in Press, January 8, 2019, DOI 10.1074/jbc.RA118.005707 X Giampiero Pietrocola‡1, Giulia Nobile‡, Mariangela J. Alfeo‡, Timothy J. Foster§, Joan A. Geoghegan¶, Vincenzo De Filippisʈ, and X Pietro Speziale‡**2 From the ‡Department of Molecular Medicine, Unit of Biochemistry, University of Pavia, 27100 Pavia, Italy, the §Microbiology Department, Trinity College Dublin, Dublin 2, Ireland, the ¶Department of Microbiology, Moyne Institute of Preventive Medicine, School of Genetics and Microbiology, Trinity College, Dublin, Dublin 2, Ireland, the ʈLaboratory of Protein Chemistry and Molecular Hematology, Department of Pharmaceutical and Pharmacological Sciences, University of Padua, 36131 Padova, Italy, and the **Department of Industrial and Information Engineering, University of Pavia, 27100 Pavia, Italy Edited by Roger J. Colbran Staphylococcus aureus is a Gram-positive bacterium that can tizing pneumoniae (1, 2). Strains that are resistant to multiple Downloaded from cause both superficial and deep-seated infections. Histones antibiotics are a major problem in healthcare settings in devel- released by neutrophils kill bacteria by binding to the bacterial oped countries (3). These are referred to as hospital-associated cell surface and causing membrane damage. We postulated that methicillin-resistant S. aureus (MRSA)3 and occur in individu- cell wall–anchored proteins protect S. aureus from the bacteri- als with pre-disposing risk factors, such as surgical wounds and cidal effects of histones by binding to and sequestering histones indwelling medical devices (4, 5). -

Digestive Enzymes: the Key to Optimum Health
Education Article Digestive Enzymes: The Key to Optimum Health Digestion is essential for good health. Unlocking nutrients from foods is a complex process. In order to break down food we rely on optimal levels and function of a special set of proteins called digestive enzymes. These proteins are found in the saliva and also in the small intestines. Without the combined actions of our digestive enzymes we would simply be unable to absorb many nutrients that we need to maintain good health. This is why reduced levels of digestive enzymes can be linked to a wide-range of symptoms within the gut and beyond. Digestive enzymes, along with stomach acid, play a crucial role in the initial stages of digestion, i.e. the breaking down of the food that we eat. The main classes of human digestive enzymes include proteases, lipases and carbohydrases, which respectively break down the macronutrients protein, fats and carbohydrates. If we do not efficiently digest these foods then vital nutrients such as essential fats, vitamins, minerals and phytonutrients cannot be absorbed. What is more, undigested or partially digested food passes through into the large intestines and is fermented by the resident colonic bacteria, causing unpleasant symptoms such as bloating and flatulence and contributing to a toxic bowel. Naturopaths believe toxicity within the bowel is the root of all disease. We do not make digestive enzymes for every type of food that we eat, such as gluten and phytic acid found in some grains and cereals and lactose, a sugar found in milk. This means our bodies may find it difficult to digest these types of foods.