Reduction of Carboxylic Acids and Their Derivatives Reduction of Carboxylic Acids
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

(+)-Obafluorin, a B-Lactone Antibiotic
Illinois Wesleyan University Digital Commons @ IWU Honors Projects Chemistry 4-26-1996 Pursuit of a Chiral Amino Aldehyde Intermediate in the Synthesis of (+)-Obafluorin, a B-Lactone Antibiotic Jim Cwik '96 Illinois Wesleyan University Follow this and additional works at: https://digitalcommons.iwu.edu/chem_honproj Part of the Chemistry Commons Recommended Citation Cwik '96, Jim, "Pursuit of a Chiral Amino Aldehyde Intermediate in the Synthesis of (+)- Obafluorin, a B-Lactone Antibiotic" (1996). Honors Projects. 15. https://digitalcommons.iwu.edu/chem_honproj/15 This Article is protected by copyright and/or related rights. It has been brought to you by Digital Commons @ IWU with permission from the rights-holder(s). You are free to use this material in any way that is permitted by the copyright and related rights legislation that applies to your use. For other uses you need to obtain permission from the rights-holder(s) directly, unless additional rights are indicated by a Creative Commons license in the record and/ or on the work itself. This material has been accepted for inclusion by faculty at Illinois Wesleyan University. For more information, please contact [email protected]. ©Copyright is owned by the author of this document. Pursuit of a Chiral Amino Aldehyde Intermediate in the Synthesis of (+)-Obafluorin, a p-Lactone Antibiotic Jim Cwik Dr. Jeffrey A. Frick, Research Advisor Submitted in Partial Fulfillment of the Requirement for Research Honors in Chemistry and Chemistry 499 Illinois Wesleyan University April 26, 1996 - Table of Contents I. List of Spectral Data , 2 II. Abstract. 3 III. Background 4 IV. Introduction 6 V. -

Chapter 3.3 Special Provisions Applicable to Certain Articles Or Substances
Chapter 3.3 Special provisions applicable to certain articles or substances 3.3.1 When Column (6) of Table A of Chapter 3.2 indicates that a special provision is relevant to a substance or article, the meaning and requirements of that special provision are as set forth below. 16 Samples of new or existing explosive substances or articles may be carried as directed by the competent authorities (see 2.2.1.1.3) for purposes including: testing, classification, research and development, qual- ity control, or as a commercial sample. Explosive samples which are not wetted or desensitized shall be limited to 10 kg in small packages as specified by the competent authorities. Explosive samples which are wetted or desensitized shall be limited to 25 kg. 23 Even though this substance has a flammability hazard, it only exhibits such hazard under extreme fire conditions in confined areas. 32 This substance is not subject to the requirements of RID when in any other form. 37 This substance is not subject to the requirements of RID when coated. 38 This substance is not subject to the requirements of RID when it contains not more than 0.1% calcium carbide. 39 This substance is not subject to the requirements of RID when it contains less than 30% or not less than 90% silicon. 43 When offered for carriage as pesticides, these substances shall be carried under the relevant pesticide entry and in accordance with the relevant pesticide provisions (see 2.2.61.1.10 to 2.2.61.1.11.2). 45 Antimony sulphides and oxides which contain not more than 0.5% of arsenic calculated on the total mass are not subject to the requirements of RID. -

UNITED STATES PATENT Office PREPARATION of BETA-Akoxy MONO Carboxylcacds Frederick E
Patented July 4, 1944 UNITED STATES PATENT office PREPARATION OF BETA-AKOxY MONO CARBOXYLCACDs Frederick E. King, Akron, Ohio, assignor to The B. F. Goodrich Company, New corporation of New York . York, N. Y., a No Drawing. Application August 5, 1941, Sera No. 405,512 4 Claims. (CL 260-535) This invention relates to a novel process for at least one hydrogen atom on the alpha carbon the preparation of beta-alkoxy derivatives of atom, for example, beta-lactones of saturated monocarboxylic acids, particularly beta-alkoxy aliphatic monocarboxylic acids such as beta-hy derivatives of saturated aliphatic monocarboxylic droxypropionic acid lactone, commonly known as acids such as beta-alkoxy proponic acids, and to 5 hydracrylic acid lactone, beta-hydroxy butyric the conversion of such acids into alkyl esters of acid lactone, alpha-methyl hydracrylic acid lac alpha beta unsaturated monocarboxylic acids tone, beta-hydroxy n-valeric acid actone, beta such as the alkyl esters of acrylic and meth hydroxy alpha-methyl butyric acid lactone, al acrylic acids. - - pharethyl hydracrylic acid lactone, beta-hydroxy In a copending application Serial No. 393,671, 0 isovaleric acid lactone, beta-hydroxy n-caproic filed May 15, 1941, an economical method of pre acid lactone, beta-hydroxy alpha-methyl valeric paring lactones of beta-hydroxy Carboxylic acids acid lactone, beta-methyl beta-ethyl hydracrylic from the reaction of a ketene with a carbonyl acid lactone, alpha-methyl beta-ethylhydracrylic compound such as an aldehyde or ketone has acid lactone, alpha-propyl -

Specific Determination of Airborne Sulfates and Sulfuric Acid
Louisiana State University LSU Digital Commons LSU Historical Dissertations and Theses Graduate School 1977 Specific etD ermination of Airborne Sulfates and Sulfuric Acid. Purnendu Kumar Dasgupta Louisiana State University and Agricultural & Mechanical College Follow this and additional works at: https://digitalcommons.lsu.edu/gradschool_disstheses Recommended Citation Dasgupta, Purnendu Kumar, "Specific eD termination of Airborne Sulfates and Sulfuric Acid." (1977). LSU Historical Dissertations and Theses. 3152. https://digitalcommons.lsu.edu/gradschool_disstheses/3152 This Dissertation is brought to you for free and open access by the Graduate School at LSU Digital Commons. It has been accepted for inclusion in LSU Historical Dissertations and Theses by an authorized administrator of LSU Digital Commons. For more information, please contact [email protected]. INFORMATION TO USERS This material was produced from a microfilm copy of the original document. While the most advanced technological means to photograph and reproduce this document have been used, the quality is heavily dependent upon the quality of the original submitted. The following explanation of techniques is provided to help you understand markings or patterns which may appear on this reproduction. 1. The sign or "target" for pages apparently lacking from the document photographed is "Missing Page(s)". If it was possible to obtain the missing page(s) or section, they are spliced into the film along with adjacent pages. This may have necessitated cutting thru an image and duplicating adjacent pages to insure you complete continuity. 2. When an image on the film is obliterated with a large round black mark, it is an indication that the photographer suspected that the copy may have moved during exposure and thus cause a blurred image. -
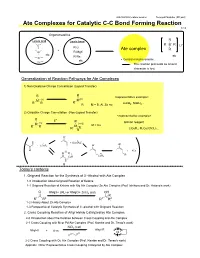
Ate Complexes for Catalytic C-C Bond Forming Reaction 1/13
2007/09/08 Literature seminer Tomoyuki Mashiko (M1 part) Ate Complexes for Catalytic C-C Bond Forming Reaction 1/13 Organometallics R Lewis acid Lewis base - B R B R Li+ R-Li + Ate complex R Al R-MgX etc R-Na etc Zn etc • Central metal is anionic. Cu The reaction proceeds as anionic character is lost. Generalization of Reaction Pathways for Ate Complexes 1) Non-Oxidative Charge Cancellation (Ligand Transfer) R R <representative example> M- (n) M(n) R R R LiAlH , NaBH .. R- M = B, Al, Zn etc 4 4. 2) Oxidative Charge Cancellation (Non-Ligand Transfer) <representative example> R + E R Gilman reagent M- (n) (n+2) R R M M = Cu R E LiCuR , R Cu(CN)Li .. R 2 2 2. O- Ⅱ + + [LiCuR2] - Ⅰ - O + LiCuR2 O Ⅰ O Li + RCu O R Ⅲ Ⅰ R CuR CuR2 0 Today's contents 1. Grignard Reaction for the Synthesis of 3°-Alcohol with Ate Complex 1-0 Introduction about Grignard Reaction of Ketone 1-1 Grignard Reaction of Ketone with Mg Ate Complex/ Zn Ate Complex (Prof. Ishihara and Dr. Hatano's work) O RMgX+ 2RLi or RMgCl+ ZnCl2 (cat) OH R R1 R2 R1 R2 1-2 History About Zn Ate Complex 1-3 Perspective of Catalytic Synthesis of 3°-alcohol with Grignard Reaction 2. Cross Coupling Reaction of Alkyl Halide Catalyzed by Ate Complex 2-0 Introduction about the Relation between Cross Coupling and Ate Complex 2-1 Cross Coupling with Ni or Pd Ate Complex (Prof. Kambe and Dr. Terao's work) NiCl2 (cat) Alkyl-R Alkyl-X + Ⅱ R-m Ni 2-2 Cross Coupling with Cu Ate Complex (Prof. -

Clark, Hobart, and Neu 1995
Waste Isolation Pilot Plant Compliance Certification Application Reference 135 Clark, D.L., D.E. Hobart, and M.P. Neu. 1995. Actinide Carbonate Complexes and Their Importance in Actinide Environmental Chemistry, Chem Revs. Vol. 95; 25-48. Submitted in accordance with 40 CFR $194.13, Submission of Reference Materials. Carera, ;., Neurcan. 3.p.. - 986 "Est~rncaonoi ?x:ier Parcrneters L'nae; Panslent anc' S:eadu Sco:z ~oi~icions,2. Unlacaness, Stc;z.:ird, and solucion ftiqo:ithrns." 9. Clark, D.L.. Floba~.D.E., Neu, M.P.. 1995 '9ctinicz Carbonate Complexes ana Thslr Irnporcccca in Ect;nide Environmencai G,~mrsny."=?em Revs. ',Jot. 05, 25-48. ON ' ; x 28.C3 398.00 Cleveland, J.M.. i 9* nGit:calRevleu of Plutonium Eov~lbrioof Environrnencal Concern. In Moueirng in Equmus S;lstms: Smrct:on, So~ubrlrtuanu tlnq of t9e Emencan Chernrcc~Societu, Pdiarnr Beacn, Fi, Series: 3521 -336. Cti~- ; x CLO.CC 220.30 . - , I. 2av1s.G.B.. Jcnnsco i 984 'Ccxxenc on Cmcamincnc Tianspor: :n fracturad PONS Media: fcr s Sjscern cS ~rciielFrcc:vres" 5y SLG~C~U,C.A., and Fmd, E.O.,' Rasc~rcesRzsecrcn, \jot. 23,i\.'o. 9. s?. : 321 - 1 322, Szpt. ., -- ? 984. 1 Qtl; I i x i 3.:: ' 48.50 , , -. - 4 Actinide Carbonate Complexes and Their Importance in Actinide Environmental Chemistry I David L. Clark,'~~~David E. Hobart,lb and Mary P. Neda Chemical Science and Technology Division, Los Alamas National Laboratory, Los Alamos, Mw Me& 87545, l?eThe Sciems DMm, Lawrence Berkeley Laboratory, BeBerky, California 94720, and UE G. T. Seabog imWe for Transactinium Scienae, I Livemre, California 94551 I Received May 16, 1994 (Revised Manuscript ReceM September 16, 1994) Table 1. -

Preparation and Properties of Aldonic Acids and Their Lactones and Basic Calcium Salts
.. U.S. Department of Commerce, Bureau of Standards RESEARCH PAPER RP613 Part of Bureau of Standards Journal of Research, Vol. 11, November 1933 PREPARATION AND PROPERTIES OF ALDONIC ACIDS AND THEIR LACTONES AND BASIC CALCIUM SALTS By Horace S. Isbell and Harriet L. Frush abstract Directions are given for the preparation of the crystalline acids and lactones derived from gluconic, mannonic, galactonic, xylonic, arabonic, and rhamnonic acids. The compositions of the basic calcium salts of mannonic, galactonic, xylonic, arabonic, rhamnonic, lactobionic, and maltobionic acids are reported and their use for the purification of sugar acids is described. The utility of dioxane as a solvent in preparing the free sugar acids and their lactones is emphasized. The method for preparing gluconic acid by crystallization from water is suitable for the manufacture of that substance in large quantity. CONTENTS I. Introduction 649 II. Gluconic acid and its derivatives 651 1. Dioxane method for preparing crystalline gluconic acid 653 2. Crystallization of gluconic acid from aqueous alcohol 653 3. Crystallization of gluconic acid from water 653 4. Preparation of gluconic 5-lactone 654 5. Preparation of gluconic 7-lactone from gluconic 5-lactone__ 655 III. Xylonic acid and its derivatives 655 1. Preparation of calcium xylonate from xylose 656 2. Preparation of xylonic 7-lactone 657 3. Preparation of basic calcium salts for analysis 657 IV. Mannonic acid and its derivatives 658 1 Calcium mannonate 658 2. Mannonic 5-lactone 659 3. Mannonic 7-lactone 659 V. Galactonic acid and its derivatives 660 1 Galactonic acid 660 2. Galactonic 7-lactone 661 VI. -

Development of Novel Methodologies in Organic Synthesis Based on Ate Complex Formation
No.171 No.171 ResearchResearch ArticleArticle Development of Novel Methodologies in Organic Synthesis Based on Ate Complex Formation Keiichi Hirano1* and Masanobu Uchiyama1,2* 1 Graduate School of Pharmaceutical Sciences, The University of Tokyo,7-3-1 Hongo, Bunkyo-ku, Tokyo 113-0033, Japan 2 Elements Chemistry Laboratory, RIKEN,2-1 Hirosawa, Wako-shi, Saitama 351-0198, Japan E-mail: [email protected]; [email protected] Abstract: We have been focusing on development of novel synthetic methodologies based on ate complex formation. By fine-tuning of coordination environments of various main-group metals (Zn, Al, and B) as well as transition metal (Cu), a wide range of carbon-carbon bond and carbon-heteroatom bond (C–I, C–O, C–N, C–H, C–Si, and C–B) formation reactions have been realized in highly regio- and chemoselective manner taking advantage of characteristics of the elements. Keywords: Ate complex, Halogen-metal exchange, Deprotonative metalation, Chemoselectivity, Regioselectivity 1. Introduction 2. Ate Complexes Ate complexes are known to be anionic organometallic Discovery of the first mono-anion type zincate, Na[ZnEt3], complexes, whose central metals have increased valence by by James A. Wanklyn dates back to 1859,1) and even di-anion accepting Lewis basic ligands to their vacant orbitals. They type zincate, Li2[ZnMe4], was already reported by Dallas T. are attractive chemical species offering tunable reactivity due Hurd in 1948.2) Zincates experienced a long blank after these to their flexible choices of central metals, counter cations, important findings before they started to attract attentions and coordination environments. -

A Sheffield Hallam University Thesis
Hydroboration of some organometallic systems. TOWERS, Christopher John. Available from the Sheffield Hallam University Research Archive (SHURA) at: http://shura.shu.ac.uk/20447/ A Sheffield Hallam University thesis This thesis is protected by copyright which belongs to the author. The content must not be changed in any way or sold commercially in any format or medium without the formal permission of the author. When referring to this work, full bibliographic details including the author, title, awarding institution and date of the thesis must be given. Please visit http://shura.shu.ac.uk/20447/ and http://shura.shu.ac.uk/information.html for further details about copyright and re-use permissions. Z S 2 S Z 0 8 0 Sheffield City Polytechnic Library REFERENCE ONLY R6297 ProQuest Number: 10701093 All rights reserved INFORMATION TO ALL USERS The quality of this reproduction is dependent upon the quality of the copy submitted. In the unlikely event that the author did not send a com plete manuscript and there are missing pages, these will be noted. Also, if material had to be removed, a note will indicate the deletion. uest ProQuest 10701093 Published by ProQuest LLC(2017). Copyright of the Dissertation is held by the Author. All rights reserved. This work is protected against unauthorized copying under Title 17, United States C ode Microform Edition © ProQuest LLC. ProQuest LLC. 789 East Eisenhower Parkway P.O. Box 1346 Ann Arbor, Ml 48106- 1346 HYDROBORATION OF SOME ORGANOMETALLIC SYSTEMS CHRISTOPHER JOHN TOWERS A thesis submitted to the Council for National Academic Awards in partial fulfilment of the requirement for Ph.D. -

Glucono Delta-Lactone Processing
Glucono Delta-Lactone Processing Executive Summary Glucono delta-lactone (GDL) was petitioned to be added to the National List as a tofu coagulant. It is produced by the oxidation of gluconic acid by a number of various methods. In addition to coagulation, GDL is used as an acidulant, leavening agent, and sequestrant. The NOSB was petitioned for this substance in 1995, and declined to refer it to the Technical Advisory Panel. The reviewers all considered it possible to make GDL from non-synthetic sources, although one considered certain sources and processes synthetic. All considered it to be non-agricultural. Two recommended that it be added to the National List with an annotation; one recommended that it remain off the National List. All three recommended that it be allowed for use in a made with organic (specified ingredients) claim. Of these, two supported with annotations, and one recommended no annotations for this use. Further investigation may be required to determine if sources are produced by the use of genetic engineering. Summary of Advised Recommendation 95% organic Synthetic / Non-Synthetic: Allowed or Prohibited: Suggested Annotation: Either synthetic Allowed (1) (Reviewer 1) None. or non-synthetic Prohibited (1) (Reviewer 2) Produced by microbial depending on the fermentation of carbohydrate substances source and or by enzymatic oxidation of organic manufacturing glucose process (1) (Reviewer 3).Must be produced by Non-synthetic (2) fermentation of glucose by naturally occurring microorganisms or enzymes Made with organic (70% or more organic ingredients) Agricultural / Non-Agricultural Allowed or Prohibited: Suggested Annotation: Non-agricultural (3) Allowed (3) (Reviewer 1) For tofu production only. -

Biocatalyzed Synthesis of Statins: a Sustainable Strategy for the Preparation of Valuable Drugs
catalysts Review Biocatalyzed Synthesis of Statins: A Sustainable Strategy for the Preparation of Valuable Drugs Pilar Hoyos 1, Vittorio Pace 2 and Andrés R. Alcántara 1,* 1 Department of Chemistry in Pharmaceutical Sciences, Faculty of Pharmacy, Complutense University of Madrid, Campus de Moncloa, E-28040 Madrid, Spain; [email protected] 2 Department of Pharmaceutical Chemistry, Faculty of Life Sciences, Althanstrasse 14, A-1090 Vienna, Austria; [email protected] * Correspondence: [email protected]; Tel.: +34-91-394-1823 Received: 25 February 2019; Accepted: 9 March 2019; Published: 14 March 2019 Abstract: Statins, inhibitors of 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase, are the largest selling class of drugs prescribed for the pharmacological treatment of hypercholesterolemia and dyslipidaemia. Statins also possess other therapeutic effects, called pleiotropic, because the blockade of the conversion of HMG-CoA to (R)-mevalonate produces a concomitant inhibition of the biosynthesis of numerous isoprenoid metabolites (e.g., geranylgeranyl pyrophosphate (GGPP) or farnesyl pyrophosphate (FPP)). Thus, the prenylation of several cell signalling proteins (small GTPase family members: Ras, Rac, and Rho) is hampered, so that these molecular switches, controlling multiple pathways and cell functions (maintenance of cell shape, motility, factor secretion, differentiation, and proliferation) are regulated, leading to beneficial effects in cardiovascular health, regulation of the immune system, anti-inflammatory and immunosuppressive properties, prevention and treatment of sepsis, treatment of autoimmune diseases, osteoporosis, kidney and neurological disorders, or even in cancer therapy. Thus, there is a growing interest in developing more sustainable protocols for preparation of statins, and the introduction of biocatalyzed steps into the synthetic pathways is highly advantageous—synthetic routes are conducted under mild reaction conditions, at ambient temperature, and can use water as a reaction medium in many cases. -

An Investigation Into the Synthesis and Characterisation of Metal
An Investigation into the Synthesis and Characterisation of Metal Borohydrides for Hydrogen Storage by Daniel Thomas Reed A thesis submitted to the University of Birmingham For the degree of Doctor of Philosophy School of Metallurgy and Materials College of Engineering and Physical Sciences University of Birmingham October 2009 University of Birmingham Research Archive e-theses repository This unpublished thesis/dissertation is copyright of the author and/or third parties. The intellectual property rights of the author or third parties in respect of this work are as defined by The Copyright Designs and Patents Act 1988 or as modified by any successor legislation. Any use made of information contained in this thesis/dissertation must be in accordance with that legislation and must be properly acknowledged. Further distribution or reproduction in any format is prohibited without the permission of the copyright holder. SYNOPSIS With relatively high gravimetric and volumetric hydrogen storage capacities, borohydride compounds are being investigated for their potential use as hydrogen storage media. A study has been made into the mechanical milling of metal chlorides with sodium borohydride to try to form homoleptic borohydrides. Various characterisation techniques have been used to characterise the composition and microstructure of the samples, and to monitor in-situ the thermal decomposition processes. It was found that rather than homoleptic borohydrides (such as Zn(BH4)2 or Mg(BH4)2), complex borohydrides of the form AM2(BH4)5 and AM(BH4)3 (where A = Li or Na and M = Zn, Mg or Ca) tend to form. Mechanical milling of zinc chloride with sodium borohydride resulted in the formation of a covalent complex NaZn2(BH4)5.