Effect of Substituents of Cerium Pyrazolates and Pyrrolates on Carbon Dioxide Activation
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Applicability of Electride Materials for Hollow Cathodes
Applicability of electride materials for hollow cathodes IEPC-2019-A-604 Presented at the 36th International Electric Propulsion Conference University of Vienna, Austria September 15-20, 2019 Malina Reitemeyer∗, Daniel Zsch¨atzschy, Kristof Holstez, Limei Chenx and Peter J. Klar{ Institute of Experimental Physics I, Justus-Liebig-University Giessen Heinrich-Buff-Ring 16, 35392 Giessen, Germany Due to its low work function, the electride C12A7:e− emits electrons at significantly lower temperatures than state of the art neutralizer materials and is a promising insert candidate for a cold-cathode design. The material's electron density and, therefore, also the emission properties are influenced by the quality of the fabrication process and surface conditions. Understanding and influencing them subsequently, is part of our current re- search. In this work, the emission characteristics of C12A7:e− as a function of the applied temperature and voltage are presented. The work function was measured as 0.52 eV ± 0.03 eV. In addition, both an intrinsic electride sample and an oxidized sample are char- acterized by Raman spectroscopy. The Raman spectra reveal an electron density of the order of 1019cm−3 to 1020cm−3 for the electride and correspond to the spectrum of the ion conducting C12A7:O2− in case of the oxidized sample. Nomenclature AG = general Richardson constant d = distance between anode and cathode e = elementary charge E = electric field I = extracted current IR = zero field current J = extracted current density JR = zero field current density -

Specific Determination of Airborne Sulfates and Sulfuric Acid
Louisiana State University LSU Digital Commons LSU Historical Dissertations and Theses Graduate School 1977 Specific etD ermination of Airborne Sulfates and Sulfuric Acid. Purnendu Kumar Dasgupta Louisiana State University and Agricultural & Mechanical College Follow this and additional works at: https://digitalcommons.lsu.edu/gradschool_disstheses Recommended Citation Dasgupta, Purnendu Kumar, "Specific eD termination of Airborne Sulfates and Sulfuric Acid." (1977). LSU Historical Dissertations and Theses. 3152. https://digitalcommons.lsu.edu/gradschool_disstheses/3152 This Dissertation is brought to you for free and open access by the Graduate School at LSU Digital Commons. It has been accepted for inclusion in LSU Historical Dissertations and Theses by an authorized administrator of LSU Digital Commons. For more information, please contact [email protected]. INFORMATION TO USERS This material was produced from a microfilm copy of the original document. While the most advanced technological means to photograph and reproduce this document have been used, the quality is heavily dependent upon the quality of the original submitted. The following explanation of techniques is provided to help you understand markings or patterns which may appear on this reproduction. 1. The sign or "target" for pages apparently lacking from the document photographed is "Missing Page(s)". If it was possible to obtain the missing page(s) or section, they are spliced into the film along with adjacent pages. This may have necessitated cutting thru an image and duplicating adjacent pages to insure you complete continuity. 2. When an image on the film is obliterated with a large round black mark, it is an indication that the photographer suspected that the copy may have moved during exposure and thus cause a blurred image. -

Interplay of Anionic Quasi-Atom and Interstitial Point Defects In
Interplay of anionic quasi-atom and interstitial point defects in electrides: abnormal interstice occupation and colossal charge state of point defects in dense FCC-lithium L. Zhang,† Q. Wu,† S. Li,† Y. Sun,† X. Yan,†, ‡ Y. Chen,+ and Hua Y. Geng*, †, § †National Key Laboratory of Shock Wave and Detonation Physics, Institute of Fluid Physics, CAEP, P.O. Box 919-102, Mianyang, Sichuan 621900, P.R. China ‡Jiangxi University of Science and Technology, Ganzhou, Jiangxi 341000, P.R. China +Fracture and Reliability Research Institute, School of Engineering, Tohoku University 6-6-01 Aramakiaoba, Aoba-ku, Sendai, 980-8579, Japan §Center for Applied Physics and Technology, HEDPS, and College of Engineering, Peking University, Beijing 100871, P.R. China Abstract: Electrides are an emerging class of materials with highly-localized electrons in the interstices of a crystal that behave as anions. The presence of these unusual interstitial quasi-atom (ISQ) electrons leads to interesting physical and chemical properties, and wide potential applications for this new class of materials. Crystal defects often have a crucial influence on the properties of materials. Introducing impurities has been proved to be an effective approach to improve the properties of a material and to expand its applications. However, the interactions between the anionic ISQs and the crystal defects in electrides are as yet unknown. Here, dense FCC-Li was employed as an archetype to explore the interplay between anionic ISQs and interstitial impurity atoms in this electride. This work reveals a strong coupling among the interstitial impurity atoms, the ISQs, and the matrix Li atoms near to the defects. -

Electride Characteristics of M2( 5-E5)
Electride Characteristics of M2(h5-E5)2 (M= Be, Mg; E= Sb5-) Prasenjit Das IIT Kharagpur: Indian Institute of Technology Kharagpur Pratim Kumar Chattaraj ( [email protected] ) IIT Kharagpur Research Article Keywords: Electride, Binuclear Sandwich Compound, Non-nuclear attractor (NNA), Electron localization function (ELF) basin, NLO properties Posted Date: February 24th, 2021 DOI: https://doi.org/10.21203/rs.3.rs-232917/v1 License: This work is licensed under a Creative Commons Attribution 4.0 International License. Read Full License Version of Record: A version of this preprint was published at Structural Chemistry on April 23rd, 2021. See the published version at https://doi.org/10.1007/s11224-021-01783-1. 5 - Electride Characteristics of M2( -E5)2 (M= Be, Mg; E= Sb5 ) Prasenjit Das,a Pratim Kumar Chattaraja,b,* a Department of Chemistry, Indian Institute of Technology Kharagpur, Kharagpur 721302, India b Department of Chemistry, Indian Institute of Technology Bombay, Mumbai, 400076, India *E-mail: [email protected] (PKC) Abstract 5 Ab initio computation is performed on the binuclear sandwich complexes, M2( -Sb5)2. Eclipsed 5 - and staggered conformations are generated due to the mode of binding by Sb5 ligand with the alkaline earth metals (Be and Mg metals). The complexes are thermodynamically stable at room temperature. The electron density descriptors and the natural bond orbital (NBO) analysis 5 5 confirmed the covalent nature of the M-M bond. Both Be2( -Sb5)2 and Mg2( -Sb5)2 complexes have one non-nuclear attractor (NNA) at the center of the M-M bond which is predicted and confirmed by the electron density analysis. -
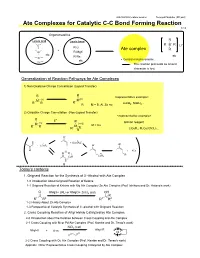
Ate Complexes for Catalytic C-C Bond Forming Reaction 1/13
2007/09/08 Literature seminer Tomoyuki Mashiko (M1 part) Ate Complexes for Catalytic C-C Bond Forming Reaction 1/13 Organometallics R Lewis acid Lewis base - B R B R Li+ R-Li + Ate complex R Al R-MgX etc R-Na etc Zn etc • Central metal is anionic. Cu The reaction proceeds as anionic character is lost. Generalization of Reaction Pathways for Ate Complexes 1) Non-Oxidative Charge Cancellation (Ligand Transfer) R R <representative example> M- (n) M(n) R R R LiAlH , NaBH .. R- M = B, Al, Zn etc 4 4. 2) Oxidative Charge Cancellation (Non-Ligand Transfer) <representative example> R + E R Gilman reagent M- (n) (n+2) R R M M = Cu R E LiCuR , R Cu(CN)Li .. R 2 2 2. O- Ⅱ + + [LiCuR2] - Ⅰ - O + LiCuR2 O Ⅰ O Li + RCu O R Ⅲ Ⅰ R CuR CuR2 0 Today's contents 1. Grignard Reaction for the Synthesis of 3°-Alcohol with Ate Complex 1-0 Introduction about Grignard Reaction of Ketone 1-1 Grignard Reaction of Ketone with Mg Ate Complex/ Zn Ate Complex (Prof. Ishihara and Dr. Hatano's work) O RMgX+ 2RLi or RMgCl+ ZnCl2 (cat) OH R R1 R2 R1 R2 1-2 History About Zn Ate Complex 1-3 Perspective of Catalytic Synthesis of 3°-alcohol with Grignard Reaction 2. Cross Coupling Reaction of Alkyl Halide Catalyzed by Ate Complex 2-0 Introduction about the Relation between Cross Coupling and Ate Complex 2-1 Cross Coupling with Ni or Pd Ate Complex (Prof. Kambe and Dr. Terao's work) NiCl2 (cat) Alkyl-R Alkyl-X + Ⅱ R-m Ni 2-2 Cross Coupling with Cu Ate Complex (Prof. -

A New Electride with Partially Filled D-Shells Padtaraporn Chanhom1,2, Kevin E
Sr3CrN3: a new electride with partially filled d-shells Padtaraporn Chanhom1,2, Kevin E. Fritz1, Lee Burton3, Jan Kloppenburg3, Yaroslav Filinchuk3, Anatol- yi Senyshin4, Maoyu Wang5, Zhenxing Feng5, Numpon Insin2, Jin Suntivich1,6, Geoffroy Hautier3 1 Materials Science and Engineering Department, Cornell University, Ithaca, NY 14850, USA 2 Department of Chemistry, Faculty of Science, Chulalongkorn University, Bangkok 10330, Thailand 3 Institute of Condensed Matter and Nanosciences, Université catholique de Louvain, Louvain-la-Neuve 1348, Belgium 4 Heinz Maier-Leibnitz Zentrum, Technische Universität München, 85748 Garching, Germany 5 School of Chemical, Biological, and Environmental Engineering, Oregon State University, Corvalis, OR 97331 USA 6 Kavli Institute at Cornell for Nanoscale Science, Cornell University, Ithaca, NY 14850, USA ABSTRACT: Electrides are ionic crystals in which the electrons prefer to occupy free space, serving as anions. Because the electrons prefer to be in the pockets, channels, or layers to the atomic orbitals around the nuclei, it has been challenging to find electrides with partially filled d-shells, since an unoc- cupied d-shell provides an energetically favourable location for the electrons to occupy. We recently predicted the existence of electrides with partially filled d-shells using high-throughput computational screening. Here, we provide an experimental support using X-ray absorption spectroscopy and X-ray and neutron diffraction to show that Sr3CrN3 is indeed an electride despite its partial d-shell configura- tion. Our findings indicate that Sr3CrN3 is the first known electride with a partially filled d-shell, in agreement with theory, which significantly broadens the criteria for the search for new electride materi- als. -

Clark, Hobart, and Neu 1995
Waste Isolation Pilot Plant Compliance Certification Application Reference 135 Clark, D.L., D.E. Hobart, and M.P. Neu. 1995. Actinide Carbonate Complexes and Their Importance in Actinide Environmental Chemistry, Chem Revs. Vol. 95; 25-48. Submitted in accordance with 40 CFR $194.13, Submission of Reference Materials. Carera, ;., Neurcan. 3.p.. - 986 "Est~rncaonoi ?x:ier Parcrneters L'nae; Panslent anc' S:eadu Sco:z ~oi~icions,2. Unlacaness, Stc;z.:ird, and solucion ftiqo:ithrns." 9. Clark, D.L.. Floba~.D.E., Neu, M.P.. 1995 '9ctinicz Carbonate Complexes ana Thslr Irnporcccca in Ect;nide Environmencai G,~mrsny."=?em Revs. ',Jot. 05, 25-48. ON ' ; x 28.C3 398.00 Cleveland, J.M.. i 9* nGit:calRevleu of Plutonium Eov~lbrioof Environrnencal Concern. In Moueirng in Equmus S;lstms: Smrct:on, So~ubrlrtuanu tlnq of t9e Emencan Chernrcc~Societu, Pdiarnr Beacn, Fi, Series: 3521 -336. Cti~- ; x CLO.CC 220.30 . - , I. 2av1s.G.B.. Jcnnsco i 984 'Ccxxenc on Cmcamincnc Tianspor: :n fracturad PONS Media: fcr s Sjscern cS ~rciielFrcc:vres" 5y SLG~C~U,C.A., and Fmd, E.O.,' Rasc~rcesRzsecrcn, \jot. 23,i\.'o. 9. s?. : 321 - 1 322, Szpt. ., -- ? 984. 1 Qtl; I i x i 3.:: ' 48.50 , , -. - 4 Actinide Carbonate Complexes and Their Importance in Actinide Environmental Chemistry I David L. Clark,'~~~David E. Hobart,lb and Mary P. Neda Chemical Science and Technology Division, Los Alamas National Laboratory, Los Alamos, Mw Me& 87545, l?eThe Sciems DMm, Lawrence Berkeley Laboratory, BeBerky, California 94720, and UE G. T. Seabog imWe for Transactinium Scienae, I Livemre, California 94551 I Received May 16, 1994 (Revised Manuscript ReceM September 16, 1994) Table 1. -

Acid-Durable Electride with Layered Ruthenium for Ammonia Synthesis: Boosting the Activity Via Cite This: Chem
Chemical Science View Article Online EDGE ARTICLE View Journal | View Issue Acid-durable electride with layered ruthenium for ammonia synthesis: boosting the activity via Cite this: Chem. Sci.,2019,10,5712 † All publication charges for this article selective etching have been paid for by the Royal Society a a b a a of Chemistry Jiang Li, ‡ Jiazhen Wu, ‡ Haiyun Wang, Yangfan Lu, Tiannan Ye, Masato Sasase, a Xiaojun Wu, *b Masaaki Kitano, *a Takeshi Inoshita a and Hideo Hosono *a Ruthenium (Ru) loaded catalysts are of significant interest for ammonia synthesis under mild reaction conditions. The B5 sites have been reported as the active sites for ammonia formation, i.e., Ru with other coordinations were inactive, which has limited the utilization efficiency of Ru metal. The implantation of Ru into intermetallic compounds is considered to be a promising approach to tune the catalytic activity and utilization efficiency of Ru. Here we report an acid-durable electride, LnRuSi (Ln ¼ La, Ce, Pr and Nd), as a B5-site-free Ru catalyst. The active Ru plane with a negative charge is selectively exposed by Creative Commons Attribution-NonCommercial 3.0 Unported Licence. chemical etching using disodium dihydrogen ethylenediaminetetraacetate (EDTA-2Na) acid, which leads to 2–4-fold enhancement in the ammonia formation rate compared with that of the original catalyst. The turnover frequency (TOF) of LnRuSi is estimated to be approximately 0.06 sÀ1, which is 600 times Received 29th March 2019 higher than that of pure Ru powder. Density functional theory (DFT) calculations revealed that the Accepted 3rd May 2019 dissociation of N2 occurs easily on the exposed Ru plane of LaRuSi. -

Development of Novel Methodologies in Organic Synthesis Based on Ate Complex Formation
No.171 No.171 ResearchResearch ArticleArticle Development of Novel Methodologies in Organic Synthesis Based on Ate Complex Formation Keiichi Hirano1* and Masanobu Uchiyama1,2* 1 Graduate School of Pharmaceutical Sciences, The University of Tokyo,7-3-1 Hongo, Bunkyo-ku, Tokyo 113-0033, Japan 2 Elements Chemistry Laboratory, RIKEN,2-1 Hirosawa, Wako-shi, Saitama 351-0198, Japan E-mail: [email protected]; [email protected] Abstract: We have been focusing on development of novel synthetic methodologies based on ate complex formation. By fine-tuning of coordination environments of various main-group metals (Zn, Al, and B) as well as transition metal (Cu), a wide range of carbon-carbon bond and carbon-heteroatom bond (C–I, C–O, C–N, C–H, C–Si, and C–B) formation reactions have been realized in highly regio- and chemoselective manner taking advantage of characteristics of the elements. Keywords: Ate complex, Halogen-metal exchange, Deprotonative metalation, Chemoselectivity, Regioselectivity 1. Introduction 2. Ate Complexes Ate complexes are known to be anionic organometallic Discovery of the first mono-anion type zincate, Na[ZnEt3], complexes, whose central metals have increased valence by by James A. Wanklyn dates back to 1859,1) and even di-anion accepting Lewis basic ligands to their vacant orbitals. They type zincate, Li2[ZnMe4], was already reported by Dallas T. are attractive chemical species offering tunable reactivity due Hurd in 1948.2) Zincates experienced a long blank after these to their flexible choices of central metals, counter cations, important findings before they started to attract attentions and coordination environments. -

High-Throughput Identification of Electrides from All Known Inorganic
Article Cite This: Chem. Mater. XXXX, XXX, XXX−XXX pubs.acs.org/cm High-Throughput Identification of Electrides from All Known Inorganic Materials Lee A. Burton, Francesco Ricci, Wei Chen, Gian-Marco Rignanese, and Geoffroy Hautier* Institute of Condensed Matter and Nanoscience, UniversitéCatholique de Louvain, 1348 Louvain-la-Neuve, Belgium *S Supporting Information ABSTRACT: In this paper, we present the results of a large- scale, high-throughput computational search for electrides among all known inorganic materials. Analyzing a database of density functional theory results on more than 60 000 compounds, we identify 65 new electride candidates and recover 4 already known. We report on all these candidates and discuss the structural and chemical factors leading to electride formation. Among these candidates, our work identifies the first partially filled 3d transition-metal-containing fi electrides Ba3CrN3 and Sr3CrN3, an unexpected nding that contravenes conventional chemistry. ■ INTRODUCTION large energy of a unbound electron.15 For example, the 2D 2+ 3− Electrides are rare ionic compounds in which an electron does electride Ca2N has two Ca cations and one N anion not occupy an atomic orbital but rather acts as an anion. Such (according to commonly held concepts of oxidation state). an electron is expected to behave differently from those The electropositive Ca cations wish to donate four electrons, occupying the valence state of standard materials, making but the anion can only accept three, leading to an excess electrides desirable as electron emitters,1 nonlinear optical electron occupying free space in the crystal structure, thereby switches,2 superconductors,3 battery anodes,4 and catalysts for satisfying all three criteria. -

Electrides and Alkalides - Comparison with Metal Solutions J
ELECTRIDES AND ALKALIDES - COMPARISON WITH METAL SOLUTIONS J. Dye To cite this version: J. Dye. ELECTRIDES AND ALKALIDES - COMPARISON WITH METAL SOLUTIONS. Journal de Physique IV Proceedings, EDP Sciences, 1991, 01 (C5), pp.C5-259-C5-282. 10.1051/jp4:1991531. jpa-00250655 HAL Id: jpa-00250655 https://hal.archives-ouvertes.fr/jpa-00250655 Submitted on 1 Jan 1991 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. JOURNAL DE PHYSfQUE IV Colloque C5, supplkment au Journal de Physique I, Vol. 1, dkcembre 1991 ELECTRIDES AND ALKALIDES - COMPARISON WITH METAL SOLUTIONS J.L. DYE Department of Chemistry and Centerfor Fundamental Matefials Research, Michigan State University, East Lansing, Michigan 48824, USA. Abstract - The similarities between species in ammonia, amine and ether solutions of the alkali metals and in crystalline alkalides and electrides is striking. In solution we can identify the species M+,olv, e-,,lV, M-, M+-e-, "e2"', and at high concentrations, e-conduction. In alkalides, electrides and mixed systems, species analogous to each of these can be found, in addition to alkali metal anion dimers, M=,olv, and chains, (M"-)". Since the last Colloque Weyl we have determined the crystal structures and properties of 30 alkalides and 4 electrides. -

Exposing the Hidden Complexity of Stoichiometric and Catalytic Metathesis Reactions by Elucidation of Mg-Zn Hybrids
Exposing the hidden complexity of stoichiometric and catalytic metathesis reactions by elucidation of Mg-Zn hybrids Eva Hevia1, Jonathan Z. Chua, Pablo García-Álvarez, Alan R. Kennedy, and Matthew D. McCall WestCHEM, Department of Pure and Applied Chemistry, University of Strathclyde, Glasgow, UK, G1 1XL Edited by Jack Halpern, University of Chicago, Chicago, IL, and approved January 14, 2010 (received for review November 17, 2009) Studying seemingly simple metathesis reactions between ZnCl2 role in these vital, new synthetic methodologies. In fact, the num- and t BuMgCl has, surprisingly, revealed a much more complex ber of organometallic compounds combining magnesium and chemistry involving mixed magnesium-zinc compounds that could zinc within the same molecule is scarce (7, 8), contrasting with be regarded as Mg-Zn hybrids. Thus, the reaction of equimolar the numerous reports on the synthesis of mixed-metal com- t amounts of ZnCl2 and BuMgCl reveals the formation of the unpre- pounds (ates) containing an alkali-metal with either magnesium μ t cedented mixed Mg-Zn complex [ðTHFÞ4Mgð -ClÞ2Znð BuÞðClÞ] (1), (magnesiates), or zinc (zincates), that show enhanced reactivity as a result of the co-complexation of the two anticipated exchange beyond the confines of the monometallic reagents from which products of the metathesis. This magnesium zincate adopts a con- they are derived (9–11). tacted ion-pair structure, closely related to Knochel’s pioneering In situ metathetical approaches are by far the most common “Turbo” Grignard reagents. Furthermore, a second coproduct iden- vehicles of choice to prepare either these mixed magnesium-zinc tified in this reaction is the solvent-separated mixed magnesium- putative intermediates or neutral organozinc reagents.