Comparative Study on the Corrosion Inhibitive Effect of 2
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Mineral Fundort (Location) Quality Habit Size Qty
Exchange list Elmar Lackner 22.03.2016 This is my exchange/trade list for advanced Collectors. All specimens are of good quality or rarity (sometimes both). Please accept that this minerals are only exchanged with comparable minerals ! Some rare Micromounts are small, but i don't use capsules (marked with small). Some hygroscopic minerals are sealed in glass-vials (mainly from Tolbachik vulcano) Quality: A = very good, B= good, C= acceptable Habit: xx = crystals, x= crystal, (xx) = intergrown crystals, (x) = intergrown crystal, oo= no crystals Sizes (Box Size !): MM: european Micromount Box, KS1 = Box 40*35*30mm, KS2 = 58*40*35mm, KS3 = 82*58*36mm, KS4 = 82*58*63mm, KS5 = 96*80*50mm Qty.: available specimens Mineral Fundort (Location) Quality Habit Size Qty. Prices More Info Adamite Tsumeb Mine, Otjikoto, Namibia B XX KS2 1 29 € green xls (Var. Cuproadamite) Adamite Mina Ojuela, Mapimi, Durango, Mexiko A XX KS3 1 15 € rich speciomen complety covered wid pale green xls Adamite (Var. Alumo-) Serpieri Mine, Laurion, Greece A+ xx MM 1 9 € very nice blue clusters on white matrix Akanthite Fresnillo, Zacatecas, Mexico A xx KS1 1 15 € xx up to 4 mm Akanthite Freiberg Distr., Erzgebirge, Sachsen, Germany A xx MM 1 9 € xx Albite Poudrette Quarry, MSH, Quebec, Kanada A+ XX KS4 1 19 € very rich specimen, pale pink crystals up to 5mm Allanpringite (Typl.) Grube Mark, Essershausen, Hessen, Germany B+ xx KS1 1 15 € rare one locality mineral, with Kakoxen and Wavellite Aluminopyracmonite (Typl.) La Fossa Crater, Vulcano, Sizilien, Italy B xx KS1, MM -

Angol-Magyar.Pdf
MINEROFIL KISKÖNYVTÁR V. Fehér Béla ÁSVÁNYKALAUZ 2. -

Shin-Skinner January 2018 Edition
Page 1 The Shin-Skinner News Vol 57, No 1; January 2018 Che-Hanna Rock & Mineral Club, Inc. P.O. Box 142, Sayre PA 18840-0142 PURPOSE: The club was organized in 1962 in Sayre, PA OFFICERS to assemble for the purpose of studying and collecting rock, President: Bob McGuire [email protected] mineral, fossil, and shell specimens, and to develop skills in Vice-Pres: Ted Rieth [email protected] the lapidary arts. We are members of the Eastern Acting Secretary: JoAnn McGuire [email protected] Federation of Mineralogical & Lapidary Societies (EFMLS) Treasurer & member chair: Trish Benish and the American Federation of Mineralogical Societies [email protected] (AFMS). Immed. Past Pres. Inga Wells [email protected] DUES are payable to the treasurer BY January 1st of each year. After that date membership will be terminated. Make BOARD meetings are held at 6PM on odd-numbered checks payable to Che-Hanna Rock & Mineral Club, Inc. as months unless special meetings are called by the follows: $12.00 for Family; $8.00 for Subscribing Patron; president. $8.00 for Individual and Junior members (under age 17) not BOARD MEMBERS: covered by a family membership. Bruce Benish, Jeff Benish, Mary Walter MEETINGS are held at the Sayre High School (on Lockhart APPOINTED Street) at 7:00 PM in the cafeteria, the 2nd Wednesday Programs: Ted Rieth [email protected] each month, except JUNE, JULY, AUGUST, and Publicity: Hazel Remaley 570-888-7544 DECEMBER. Those meetings and events (and any [email protected] changes) will be announced in this newsletter, with location Editor: David Dick and schedule, as well as on our website [email protected] chehannarocks.com. -

The New IMA List of Minerals – a Work in Progress – Updated: March 2020
The New IMA List of Minerals – A Work in Progress – Updated: March 2020 In the following pages of this document a comprehensive list of all valid mineral species is presented. The list is distributed (for terms and conditions see below) via the web site of the Commission on New Minerals, Nomenclature and Classification of the International Mineralogical Association, which is the organization in charge for approval of new minerals, and more in general for all issues related to the status of mineral species. The list, which will be updated on a regular basis, is intended as the primary and official source on minerals. Explanation of column headings: Name : it is the presently accepted mineral name (and in the table, minerals are sorted by name). CNMMN/CNMNC approved formula : it is the chemical formula of the mineral. IMA status : A = approved (it applies to minerals approved after the establishment of the IMA in 1958); G = grandfathered (it applies to minerals discovered before the birth of IMA, and generally considered as valid species); Rd = redefined (it applies to existing minerals which were redefined during the IMA era); Rn = renamed (it applies to existing minerals which were renamed during the IMA era); Q = questionable (it applies to poorly characterized minerals, whose validity could be doubtful). IMA No. / Year : for approved minerals the IMA No. is given: it has the form XXXX-YYY, where XXXX is the year and YYY a sequential number; for grandfathered minerals the year of the original description is given. In some cases, typically for Rd and Rn minerals, the year may be followed by s.p. -

Technological Challenges of Phosphorus Removal in High-Phosphorus Ores: Sustainability Implications and Possibilities for Greener Ore Processing
sustainability Review Technological Challenges of Phosphorus Removal in High-Phosphorus Ores: Sustainability Implications and Possibilities for Greener Ore Processing Stanley Udochukwu Ofoegbu 1,2 1 Centre for Mechanical Technology and Automation (TEMA), Department of Mechanical Engineering, University of Aveiro, Campus Universitário de Santiago, 3810-193 Aveiro, Portugal; [email protected]; Tel.: +351-915-2486-42 2 Department of Materials and Ceramic Engineering, CICECO-Aveiro Institute of Materials, University of Aveiro, Campus Universitário de Santiago, 3810-193 Aveiro, Portugal Received: 28 October 2019; Accepted: 26 November 2019; Published: 29 November 2019 Abstract: With the present rates of iron ore consumption, currently unusable, high-phosphorus iron ore deposits are likely to be the iron ores of the future as higher-grade iron ore reserves are depleted. Consequently, the design and timely development of environmentally-benign processes for the simultaneous beneficiation of high-phosphorus iron ores and phosphorus recovery, currently a technological challenge, might soon become a sustainability challenge. To stimulate interest in this area, phosphorus adsorption and association in iron oxides/hydroxyoxides, and current efforts at its removal, have been reviewed. The important properties of the most relevant crystalline phosphate phases in iron ores are highlighted, and insights provided on plausible routes for the development of sustainable phosphorus recovery solutions from high-phosphorus iron ores. Leveraging literature -

Studium Sekundárních Fosfátů S Allanpringitem a Tvrdýitem Z Opuštěného Ložiska Železných Rud Krušná Hora U Berouna (Česká Republika)
Bull Mineral Petrolog 27, 2, 2019. ISSN 2570-7337 (print); 2570-7345 (online) 231 PŮVODNÍ PRÁCE/ORIGINAL PAPER Studium sekundárních fosfátů s allanpringitem a tvrdýitem z opuštěného ložiska železných rud Krušná hora u Berouna (Česká republika) A study of secondary phosphates with allanpringite and tvrdýite from the abandoned iron deposit Krušná hora near Beroun (Czech Republic) Luboš VRtiška1,2)*, Jiří SeJkora2) a radana Malíková2) 1)Ústav geologických věd, Přírodovědecká fakulta, Masarykova univerzita, Kotlářská 267/2, 611 37 Brno; *e-mail: [email protected] 2)Mineralogicko-petrologické oddělení, Národní muzeum, Cirkusová 1740, 193 00 Praha 9 - Horní Počernice vrtiška l, SeJkora J, Malíková r (2019) Studium sekundárních fosfátů s allanpringitem a tvrdýitem z opuštěného lo- žiska železných rud Krušná hora u Berouna (Česká republika). Bull Mineral Petrolog 27(2): 231-246 ISSN 2570-7337 Abstract During the revision of old localities represented by historical samples in the mineralogical collection of the National Museum in Prague an interesting association of phosphate minerals in iron ores from abandoned iron deposit Krušná hora in Central Bohemia was discovered. The locality Krušná hora is situated about 12 km NW of Beroun (30 km WSW of Prague, Czech Republic) in an area of the Ordovician sedimentary rocks of the Teplá-Barrandian unit. Phosphates are bound to cracks and cavities in iron ores (mainly hematite). Most frequently observed phosphate minerals are: variscite and strengite, which occur there as white to transparent concentric aggregates up to 2 mm in size; fluorwavellite which forms white needle crystals up to 4 mm with contents of F up to 1.33 apfu and yellow to orange cacoxenite which occurs there as radial and concentric aggregates up to 1.5 mm in size with content of Al up to 5.80 apfu. -

IMA–CNMNC Approved Mineral Symbols
Mineralogical Magazine (2021), 85, 291–320 doi:10.1180/mgm.2021.43 Article IMA–CNMNC approved mineral symbols Laurence N. Warr* Institute of Geography and Geology, University of Greifswald, 17487 Greifswald, Germany Abstract Several text symbol lists for common rock-forming minerals have been published over the last 40 years, but no internationally agreed standard has yet been established. This contribution presents the first International Mineralogical Association (IMA) Commission on New Minerals, Nomenclature and Classification (CNMNC) approved collection of 5744 mineral name abbreviations by combining four methods of nomenclature based on the Kretz symbol approach. The collection incorporates 991 previously defined abbreviations for mineral groups and species and presents a further 4753 new symbols that cover all currently listed IMA minerals. Adopting IMA– CNMNC approved symbols is considered a necessary step in standardising abbreviations by employing a system compatible with that used for symbolising the chemical elements. Keywords: nomenclature, mineral names, symbols, abbreviations, groups, species, elements, IMA, CNMNC (Received 28 November 2020; accepted 14 May 2021; Accepted Manuscript published online: 18 May 2021; Associate Editor: Anthony R Kampf) Introduction used collection proposed by Whitney and Evans (2010). Despite the availability of recommended abbreviations for the commonly Using text symbols for abbreviating the scientific names of the studied mineral species, to date < 18% of mineral names recog- chemical elements -

Ernie New.Fp5
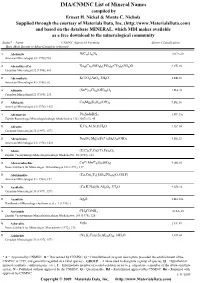
IMA/CNMNC List of Mineral Names compiled by Ernest H. Nickel & Monte C. Nichols Supplied through the courtesy of Materials Data, Inc. (http://www.MaterialsData.com) and based on the database MINERAL, which MDI makes available as a free download to the mineralogical community Status* Name CNMMC Approved Formula Strunz Classification Best, Most Recent or Most Complete reference. A Abelsonite NiC£¡H£¢N¤ 10.CA.20 American Mineralogist 63 (1978) 930 A Abenakiite-(Ce) Na¢¦Ce¦(SiO£)¦(PO¤)¦(CO£)¦(SO¢)O 9.CK.10 Canadian Mineralogist 32 (1994), 843 G Abernathyite K(UO¢)AsO¤•3H¢O 8.EB.15 American Mineralogist 41 (1956), 82 A Abhurite (SnÀÈ)¢¡Cl¡¦(OH)¡¤O¦ 3.DA.30 Canadian Mineralogist 23 (1985), 233 D Abkhazite Ca¢Mg¥Si¨O¢¢(OH)¢ American Mineralogist 63 (1978), 1023 D Abrazite K,Ca,Al,Si,O,H¢O Canadian Mineralogist 35 (1997), 1571 D Abriachanite Na¢(Fe,Mg)£(FeÁÈ)¢Si¨O¢¢(OH)¢ American Mineralogist 63 (1978), 1023 D Absite (U,Ca,Y,Ce)(Ti,Fe)¢O¦ Zapiski Vsesoyuznogo Mineralogicheskogo Obshchestva 92 (1963), 113 A Abswurmbachite CuÀÈ(MnÁÈ)¦O¨(SiO¤) 9.AG.05 Neues Jahrbuch für Mineralogie, Abhandlungen 163 (1991), 117 D Abukumalite (Ca,Ce)¢Y£(SiO¤,PO¤)£(O,OH,F) American Mineralogist 51 (1966), 152 D Acadialite (Ca,K,Na)(Si,Al)£O¦•3H¢O Canadian Mineralogist 35 (1997), 1571 G Acanthite Ag¢S 2.BA.35 Handbook of Mineralogy (Anthony et al.), 1 (1990), 1 A Acetamide CH£CONH¢ 10.AA.20 Zapiski Vsesoyuznogo Mineralogicheskogo Obshchestva 104 (1975), 326 G Achavalite FeSe 2.CC.05 Neues Jahrbuch für Mineralogie, Monatshefte (1972), 276 D Achiardite (Na,K,Ca)¥(Si,Al)¢¤O¤¨•14H¢O Canadian Mineralogist 35 (1997), 1571 D Achlusite Na,K,Al,Si,O(?) Canadian Mineralogist 36 (1998), 905 D Achrematite Pb,Mo,As,O,Cl American Mineralogist 62 (1977), 170 * A = Approved by CNMNC, D = Discredited by CNMNC, G = Grandfathered (original description preceded the establishment of the CNMNC in 1959, and generally regarded as a valid species) , GROUP = A name used to designate a group of species, H = Hypothetical mineral (synthetic, anthropogenic, etc.), I = Intermediate member of a solid-solution series (e.g. -

Glossary of Obsolete Mineral Names
a (anorthic) = triclinic system, CM 25, 353 (1987). A.1 = kaolinite or saggar-clay, Robertson 6 (1954). AII = anhydrite, AM 91, 619 (2006). aabam = lead, de Fourestier 1 (1999). aanerödite = samarskite-(Y), AM 9, 62 (1924). aanerodite = samarskite-(Y), Aballain et al. 1 (1968). aannerödite = samarskite-(Y), Clark 3 (1993). aannerodite = samarskite-(Y), Aballain et al. 1 (1968). aarite = As-rich breithauptite, Dana 6th, 71 (1892). aastrophyllite = astrophyllite, AM 83, 190 (1998). abacus = quartz-mogánite mixed-layer, Hintze I.2, 1494 (1905). Abacus-Steine = quartz-mogánite mixed-layer, Chudoba RI, 3 (1939); [I.2.1494]. Abaeté = 238 ct. diamond, Cornejo & Bartorelli 213 (2010). abakus stone = quartz-mogánite mixed-layer, Bukanov 135 (2006). abakusz-kő = quartz-mogánite mixed-layer, László 138 (1995). abbecasite = asbecasite, AM 54, 330 (1969). Abchasit = tremolite, Chudoba EII, 1 (1954). Abendsmaragd = gem forsterite, László 247 (1995). abernatheyite = abernathyite, AM Index 41-50, 10 (1968). Abeston = actinolite or chrysotile, de Fourestier 1 (1999). abhazit = tremolite, László 1 (1995). Abichit = clinoclase, Clark 3 (1993). abkhazite = tremolite, AM 63, 1049 (1978). ablick clay = halloysite-7Å, MM 26, 334 (1943). ablikite = halloysite-7Å, MM 26, 334 (1943). ablygonite = amblygonite or montebrasite, de Fourestier 1 (1999). ablykite = halloysite-7Å, AM 25, 768 (1940). A.B.M. = C-rich kaolinite + illite ?, Robertson 7 (1954). abnormal-bornite = bornite ?, AM 56, 1895 (1971). abnormaler Psilomelan = romanèchite, Doelter III.2, 872 (1926). abracita = gismondine, de Fourestier 1 (1999). Abrahamgottlobwernerit = hypothetical mineral, LAP 21(6), 50 (1996). abraum salts = carnallite + sylvite + kieserite, Dana 6th, 933 (1892). Abraumsalze = carnallite + sylvite + kieserite, Dana 6th, 933 (1892). abrazite = gismondine or phillipsite-K, Clark 3 (1993). -
IMA/CNMNC List of Mineral Names
IMA/CNMNC List of Mineral Name s compiled by Ernest H. Nickel & Monte C. Nichols Supplied through the courtesy of Materials Data, Inc. (http://www.MaterialsData.com) and based on the database MINERAL, which MDI makes available as a free download to the mineralogical community Status* Name CNMNC Approved Formula Strunz Classification Best, Most Recent or Most Complete reference. A Abelsonite NiC£¡H£¢N¤ 10.CA.20 American Mineralogist 63 (1978) 930 A Abenakiite-(Ce) Na¢¦Ce¦(SiO£)¦(PO¤)¦(CO£)¦(SO¢)O 9.CK.10 Canadian Mineralogist 32 (1994), 843 G Abernathyite K(UO¢)AsO¤•3H¢O 8.EB.15 American Mineralogist 41 (1956), 82 A Abhurite (SnÀÈ)¢¡Cl¡¦(OH)¡¤O¦ 3.DA.30 Canadian Mineralogist 23 (1985), 233 D Abkhazite Ca¢Mg¥Si¨O¢¢(OH)¢ 9.DE.10 American Mineralogist 63 (1978), 1023 A Abramovite Pb¢SnInBiS§ 2.HF.25a Zapiski Rossiiskogo Mineralogicheskogo Obshchetstva 136 (2007) (5), 45 D Abrazite K,Ca,Al,Si,O,H¢O 9.GC.05 Canadian Mineralogist 35 (1997), 1571 D Abriachanite Na¢(Fe,Mg)£(FeÁÈ)¢Si¨O¢¢(OH)¢ 9.DE.25 American Mineralogist 63 (1978), 1023 D Absite (U,Ca,Y,Ce)(Ti,Fe)¢O¦ Zapiski Vsesoyuznogo Mineralogicheskogo Obshchestva 92 (1963), 113 A Abswurmbachite CuÀÈ(MnÁÈ)¦O¨(SiO¤) 9.AG.05 Neues Jahrbuch für Mineralogie, Abhandlungen 163 (1991), 117 D Abukumalite (Ca,Ce)¢Y£(SiO¤,PO¤)£(O,OH,F) American Mineralogist 51 (1966), 152 D Acadialite (Ca,K,Na)(Si,Al)£O¦•3H¢O 9.GD.10 Canadian Mineralogist 35 (1997), 1571 G Acanthite Ag¢S 2.BA.30a Handbook of Mineralogy (Anthony et al.), 1 (1990), 1 A Acetamide CH£CONH¢ 10.AA.20 Zapiski Vsesoyuznogo Mineralogicheskogo -

IMA Master List
The New IMA List of Minerals – A Work in Progress – Updated: October 2013 In the following pages of this document a comprehensive list of all valid mineral species is presented. The list is distributed (for terms and conditions see below) via the web site of the Commission on New Minerals, Nomenclature and Classification of the International Mineralogical Association, which is the organization in charge for approval of new minerals, and more in general for all issues related to the status of mineral species. The list, which will be updated on a regular basis, is intended as the primary and official source on minerals. Explanation of column headings: Name: it is the presently accepted mineral name (and in the table, minerals are sorted by name). Chemical formula: it is the CNMNC-approved formula. IMA status: A = approved (it applies to minerals approved after the establishment of the IMA in 1958); G = grandfathered (it applies to minerals discovered before the birth of IMA, and generally considered as valid species); Rd = redefined (it applies to existing minerals which were redefined during the IMA era); Rn = renamed (it applies to existing minerals which were renamed during the IMA era); Q = questionable (it applies to poorly characterized minerals, whose validity could be doubtful). IMA No. / Year: for approved minerals the IMA No. is given: it has the form XXXX-YYY, where XXXX is the year and YYY a sequential number; for grandfathered minerals the year of the original description is given. In some cases, typically for Rd and Rn minerals, the year may be followed by s.p. -

Joint 5Th Central-European Mineralogical Conference and 7Th Mineral Sciences in the Carpathians Conference
Joint 5th Central-European Mineralogical Conference and 7th Mineral Sciences in the Carpathians Conference Book of Contributions and Abstracts Banská Štiavnica June 26 – 30, 2018 Comenius University in Bratislava Mineralogical Society of Slovakia (SMS) and Slovak Mining Museum (SBM) Joint 5th Central-European Mineralogical Conference and 7th Mineral Sciences in the Carpathians Conference Book of Contributions and Abstracts Scientific Board: Igor Broska (Slovak Academy of Science, Bratislava), Andreas Ertl (University of Vienna), Shah Wali Faryad (Charles University, Prague), Jozef Labuda (Slovak Mining Museum, Banská Štiavnica), Milan Novák (Masaryk University, Brno), Marian Putiš (Comenius University, Bratislava), Adam Pieczka (AGH University of Science and Technology, Kraków), Ján Spišiak (Matej Bel University, Banská Bystrica), Csaba Szabó (Eötvös Loránd University, Budapest), Sándor Szakáll (University of Miskolc) Organizers: Peter Bačík (Comenius University, Bratislava), Pavel Uher (Comenius University, Bratislava), Jana Fridrichová (Comenius University, Bratislava), Martin Ondrejka (Comenius University, Bratislava), Peter Koděra (Comenius University, Bratislava) Martin Macharík (stiavnica.sk), Tomáš Mikuš (Slovak Academy of Science, Banská Bystrica), Martin Števko (UK Mining Ventures Ltd., East Coker), Jan Cempírek (Masaryk University, Brno) Mineralogical Society of Slovakia 2018 ISBN 978-80-223-4548-4 Joint 5th Central-European Mineralogical Conference and 7th Mineral Sciences in the Carpathians Conference Editors: Martin Ondrejka