Download The
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Potato - Wikipedia, the Free Encyclopedia
Potato - Wikipedia, the free encyclopedia Log in / create account Article Talk Read View source View history Our updated Terms of Use will become effective on May 25, 2012. Find out more. Main page Potato Contents From Wikipedia, the free encyclopedia Featured content Current events "Irish potato" redirects here. For the confectionery, see Irish potato candy. Random article For other uses, see Potato (disambiguation). Donate to Wikipedia The potato is a starchy, tuberous crop from the perennial Solanum tuberosum Interaction of the Solanaceae family (also known as the nightshades). The word potato may Potato Help refer to the plant itself as well as the edible tuber. In the region of the Andes, About Wikipedia there are some other closely related cultivated potato species. Potatoes were Community portal first introduced outside the Andes region four centuries ago, and have become Recent changes an integral part of much of the world's cuisine. It is the world's fourth-largest Contact Wikipedia food crop, following rice, wheat and maize.[1] Long-term storage of potatoes Toolbox requires specialised care in cold warehouses.[2] Print/export Wild potato species occur throughout the Americas, from the United States to [3] Uruguay. The potato was originally believed to have been domesticated Potato cultivars appear in a huge variety of [4] Languages independently in multiple locations, but later genetic testing of the wide variety colors, shapes, and sizes Afrikaans of cultivars and wild species proved a single origin for potatoes in the area -

And Glycemic Load (GL) Values—2008
Revised International Table of Glycemic Index (GI) and Glycemic Load (GL) Values—2008 By David Mendosa This is the definitive table for both the glycemic index and the glycemic load. I am able to reproduce it here courtesy of the author, Professor Jennie Brand-Miller of the University of Sydney. It is based on a table in different format but no more foods published December 2008 in Diabetes Care. However, only the abstract is free online there. GI of 55 is low; GL of 10 is low. This table includes the glycemic index and glycemic load of more than 2,480 individual food items. Not all of them, however, are available in the United States. They represent a true international effort of testing around the world. The glycemic index (GI) is a numerical system of measuring how much of a rise in circulating blood sugar a carbohydrate triggers–the higher the number, the greater the blood sugar response. So a low GI food will cause a small rise, while a high GI food will trigger a dramatic spike. A list of carbohydrates with their glycemic values is shown below. A GI is 70 or more is high, a GI of 56 to 69 inclusive is medium, and a GI of 55 or less is low. The glycemic load (GL) is a relatively new way to assess the impact of carbohydrate consumption that takes the glycemic index into account, but gives a fuller picture than does glycemic index alone. A GI value tells you only how rapidly a particular carbohydrate turns into sugar. -

Potato Glossary
A Potato Glossary A Potato Glossary by Richard E. Tucker Last revised 15 Sep 2016 Copyright © 2016 by Richard E. Tucker Introduction This glossary has been prepared as a companion to A Potato Chronology. In that work, a self-imposed requirement to limit each entry to a single line forced the use of technical phrases, scientific words, jargon and terminology that may be unfamiliar to many, even to those in the potato business. It is hoped that this glossary will aid those using that chronology, and it is hoped that it may become a useful reference for anyone interested in learning more about potatoes, farming and gardening. There was a time, a century or more ago, when nearly everyone was familiar with farming life, the raising of potatoes in particular and the lingo of farming in general. They were farmers themselves, they had relatives who farmed, they knew someone who was a farmer, or they worked on a nearby farm during their youth. Then, nearly everyone grew potatoes in their gardens and sold the extra. But that was a long ago time. Now the general population is now separated from the farm by several generations. Only about 2 % of the US population lives on a farm and only a tiny few more even know anyone who lives on a farm. Words and phrases used by farmers in general and potato growers in particular are now unfamiliar to most Americans. Additionally, farming has become an increasingly complex and technical endeavor. Research on the cutting edge of science is leading to new production techniques, new handling practices, new varieties, new understanding of plant physiology, soil and pest ecology, and other advances too numerous to mention. -

Colorado Certified Seed Potatoes Crop Directory
www.coloradocertifiedpotatogrowers.com 2014 COLORADO CERTIFIED SEED POTATOES CROP DIRECTORY and FIELD INSPECTION READINGS COLORADO POTATO CERTIFICATION SERVICE San Luis Valley Research Center 0249 East Rd. 9 North Center, CO 81125 719 754-3496 FAX: 719 754-2619 COLORADO CERTIFIED POTATO GROWERS’ ASSOCIATION, INC. IN COOPERATION WITH COLORADO STATE UNIVERSITY Seed potatoes are certified in Colorado through authority granted by the Colorado State Board of Agriculture THE RULES AND REGULATIONS FOR COLORADO CERTIFIED SEED “By law and purpose, the Colorado State POTATOES, AS INCORPORATEDUniversity HEREIN,Cooperative HAVEExtension BEEN Service PROMULGATED BY THE BOARD OF GOVERNORS OFis dedicated THE CSto serveU SYSTEM all people onPURSUANT an TO LEGISLATION equal and nondiscriminatory basis.” ENACTED BY THE COLORADO GENERAL ASSEMBLY, AS INCORPORATED IN SESSION LAWS, 1929, CHAPTER 59, PAGE 188, AND SESSION LAWS, 1933, CHAPTER 25, PAGE 173. SINCE THE USE, CROP, YIELDS, OR QUALITY OF CERTIFIED SEED POTATOES IS BEYOND THE CONTROL OF THE PRODUCER, THE SELLER, THE INSPECTOR, THE COLORADO POTATO GROWERS’ ASSN., INC. OR THE BOARD OF GOVERNORS OF THE CSU SYSTEM, NO WARRANTY OF ANY KIND, EXPRESS OR IMPLIED, INCLUDING MERCHANTABILITY, WHICH EXTENDS BEYOND THE DESCRIPTION ON TAG OR BULK CERTIFICATE IS MADE CONCERNING THE PERFORMANCE OR QUALITY OF THESE SEED POTATOES. BY ACCEPTANCE OF THESE SEED POTATOES, BUYER EXPRESSLY AGREES THAT ITS EXCLUSIVE REMEDY FOR BREACH OF ANY WARRANTY SHALL BE LIMITED IN ALL EVENTS TO A RETURN OF THE PURCHASE PRICE OF THE SEED. “By law and purpose, the Colorado State University Cooperative Extension Service is dedicated to serve all people on an equal and nondiscriminatory basis.” THE POTATO CERTIFICATION SERVICE AND THE COLORADO CERTIFIED SEED POTATO GROWERS SINCERELY HOPE THE 2014 SEED POTATO DIRECTORY WILL BE HELPFUL TO SEED BUYERS. -

State of Maine Foundation / Certified Seed Potatoes As Determined by the Florida Test 2012 - 2013" (2013)
Maine State Library Digital Maine Animal and Plant Health Documents Agriculture, Conservation and Forestry Spring 2013 State of Maine Foundation / Certified eedS Potatoes as Determined by the Florida Test 2012 - 2013 Maine Department of Agriculture, Conservation and Forestry Follow this and additional works at: https://digitalmaine.com/aph_docs Recommended Citation Maine Department of Agriculture, Conservation and Forestry, "State of Maine Foundation / Certified Seed Potatoes as Determined by the Florida Test 2012 - 2013" (2013). Animal and Plant Health Documents. 3. https://digitalmaine.com/aph_docs/3 This Text is brought to you for free and open access by the Agriculture, Conservation and Forestry at Digital Maine. It has been accepted for inclusion in Animal and Plant Health Documents by an authorized administrator of Digital Maine. For more information, please contact [email protected]. STATE OF MAINE Foundation/Certified Seed Potatoes As determined by the Florida Test 20122012---- 202020120 1113333 The readings in the Foundation List do not exceed tolerances for Maine Foundation Seed Potatoes. (0.5% total virus and produced on farms where no Ring Rot was found in 2012.) Included in this book are those seed lots that met the tolerances for Maine Certified Seed Potatoes. Printed under appropriation number 014 01A 7871 012 Maine Department of Agriculture, Conservation & Forestry Food and Rural Resources Division of Animal & Plant Health 744 Main Street, Suite 9 Presque Isle, Maine 04769 Contents Division of Animal & Plant Health Florida -

Seed Potatoes Northwest 4620 Churchill Rd., Manhattan, 8300 Hwy
December 2015 Country 2015-16 Seed Potato POTATO COUNTRY 6777 NE Vinings Way #1324, Hillsboro, OR 97124 CHANGE SERVICE REQUESTED Directory Vol. 31 No. 8 www.potatocountry.com Columbia Publishing 6777 NE Vinings Way #1324 Table of Contents Country Hillsboro, Oregon 97124 Phone: (509) 248-2452 December 2015 Editorial Board 4 2015-2016 Seed Potato Directory Washington State Chris Voigt Executive Director 4 Montana Potato Commission 8 Oregon (509) 765-8845 10 Washington 11 Idaho 16 Alaska Oregon Potato Commission 16 California Bill Brewer 16 Colorado (503) 239-4763 Executive Director Insect Identification Quiz:Andy Jensen, Ph.D., regional re- 18 Maine search director, Washington, Idaho and Oregon potato commissions. 23 Michigan Disease Identification Quiz:Dr. Jeff Miller, plant pathologist 24 Minnesota and president and CEO of Miller Research, Rupert, Idaho. 26 Nebraska/Wyoming Potato Country Contacts 26 New York Denise Keller ....................... [email protected] Editor 27 North Dakota J. Mike Stoker [email protected] Publisher / Advertising Manager 29 Wisconsin Jeraleh Kastner ................. [email protected] 30 Alberta Production / Circulation Manager D. Brent Clement [email protected] 33 British Columbia Consultant EDITORIAL INFORMATION 34 Manitoba Potato Country is interested in newsworthy material related to potato production and marketing. Contributions from all segments of the industry 35 New Brunswick are welcome. Submit news releases, new product submissions, stories and photos via email to: [email protected]. 37 Prince Edward Island ADVERTISING SALES For information about advertising rates, mechanics, deadlines, copy submis- sion, mailing, contract conditions and other information, call Mike Stoker at 39 Quebec (509) 949-1620 or email [email protected]. -

Tissue Culture Cultivars
Cultivar/Clone Other Names Source Tuber Shape Maturity Skin Flesh Flower Protected By 04 6089.1 Norika Higgins 05 6261.1 Norika Higgins 05 6556.1 Norika Higgins 05.6700.1 IPB Higgins 05-152 IPB WHITE Higgins Farms 06 6860.2 Norika Higgins 06.6065.2 IPB Higgins 06-363 IPB WHITE Higgins Farms 08 6675.3 Norika Higgins 08 6840.1 Norika Higgins 08.6735.1 IPB Higgins 08-181R IPB RED YELLOW Higgins Farms 08-407 IPB YELLOW Higgins Farms 735 102-09 Norika Higgins 831 211-10 Maryann Norika Higgins AC Glacier Chip CANADA AC Maple Gold CANADA AC99330-1P/Y Midnight Moon CO ROUND MEDIUM PURPLE YELLOW SLVRC Accumulator W2324-1 WI Round white white WI ADIB Norika Higgins Adirondack Blue NY ROUND EARLY/MED PURPLE PURPLE WHITE Alasclear LONG WHITE WHITE Alaska Frostless ROUND WHITE WHITE Albatros Norika OBLONG YELLOW YELLOW Higgins Farms Alegria US 476-94 Norika OVAL MEDIUM YELLOW YELLOW Higgins Farms All Blue OR LONG LATE BLUE BLUE LT. BLUE All Red WA OBLONG EARLY/MED RED RED DARK PINK Allora Norika Oval/Oblong Med/Early YELLOW YELLOW Higgins Farms Alpha CANADA OVAL LATE YELLOW YELLOW RED PURPLE Alturas A82360-7 ID LONG LATE LIGHT RU WHITE U of Idaho Alwara Q44276 USDA OVAL EARLY RED YELLOW Amandine CSS Germicopa AmaRosa POR01PG22-1 FINGERLING MEDIUM RED RED Oregon Anoka ROUND WHITE WHITE Dakota AOND95249-1 ND LONG LATE RUSSET WHITE White NDSU Trailblazer Aquila Asterix OBLONG LATE RED YELLOW RED-VIOLET HZPC ATC00293-1W/Y CO OBLONG MEDIUM WHITE YELLOW Purple SLVRC Athlete CSS OVAL EARLY YELLOW YELLOW Purple Agrico Atlantic CANADA OBLONG MEDIUM BUFF WHITE LAVENDER ATTX05175s-1R/Y TX Texas ATTX07042-3W TX TX ATTX98444-16R/Y CO OBLONG RED YELLOW texas ATX06264S-4R/Y TX TEXAS A&M Aussie 96 Austrian Crescent NY FINGER MEDIUM WHITE YELLOW Pink John Mara (Higgins Avalanche^^ IPB OBLONG MEDIUM WHITE WHITE Farms, Grimway) Bake-King Baltic Fire Norika Norika Baltic Rose 32 213-06 Norika OBLONG PINK YELLOW Norika MED. -

WO 2016/007985 Al 21 January 2016 (21.01.2016) P O P C T
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2016/007985 Al 21 January 2016 (21.01.2016) P O P C T (51) International Patent Classification: (81) Designated States (unless otherwise indicated, for every A01P 21/00 (2006.01) A01N 43/653 (2006.01) kind of national protection available): AE, AG, AL, AM, A01G 1/00 (2006.01) A01N 43/54 (2006.01) AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, (21) Number: International Application DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, PCT/AU2015/000393 HN, HR, HU, ID, IL, IN, IR, IS, JP, KE, KG, KN, KP, KR, (22) International Filing Date: KZ, LA, LC, LK, LR, LS, LU, LY, MA, MD, ME, MG, 8 July 2015 (08.07.2015) MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, OM, PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SA, SC, (25) Filing Language: English SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, TN, (26) Publication Language: English TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. (30) Priority Data: (84) Designated States (unless otherwise indicated, for every 2014902720 15 July 2014 (15.07.2014) AU kind of regional protection available): ARIPO (BW, GH, GM, KE, LR, LS, MW, MZ, NA, RW, SD, SL, ST, SZ, (71) Applicant: SIMPLOT AUSTRALIA PTY LTD TZ, UG, ZM, ZW), Eurasian (AM, AZ, BY, KG, KZ, RU, [AU/AU]; Chifley Business Park, 2 Chifley Drive, TJ, TM), European (AL, AT, BE, BG, CH, CY, CZ, DE, Mentone, Victoria 31 4 (AU). -

Proceedings of the Fourteenth Euroblight Workshop PPO SPECIAL REPORT NO.PPO 16 SPECIAL REPORT
PPO 568 PPO LIMASSOL - CYPRUS 12-15 MAY 2013 PPO SPECIAL REPORT NO. 16 Proceedings of the fourteenth EuroBlight Workshop PPO SPECIAL REPORT NO. 16 SPECIAL REPORT 16 PPO NO. AARHUS AU UNIVERSITY 15429_PPO_omslag_Euroblight_raport16.indd 1-3 3-2-2014 17:57:08 PPO-Special Report no. 16 January 2014 H.T.A.M. Schepers (editor) PPO-Special Report from no. 8 onwards: ISSN 1569 - 321X PPO-Special Report from no’s. 1,2,3,4,5,6,7: ISSN 1386 - 3126 Applied Plant Research (Praktijkonderzoek Plant & Omgeving, PPO) part of Wageningen UR, is the ultimate knowledge institute for arable Farming, Multifunctional Agriculture and Field Production of Vegatables. January 2014 - PPO no. 568 Colofon © 2014 Wageningen, DLO Foundation All rights reserved. No part of this publication may be reproduced, stored in a retrieval system or transmitted, in any form or by any means, electronic, mechanical, photocopying, recording or otherwise, without the prior written permission of the DLO Foundation, Praktijkonderzoek Plant & Omgeving (Applied Plant Research), Business Unit Arable Farming, Multifunctional Agriculture and Field Production of Vegatables. The Foundation DLO is not responsible for any damage caused by using the content of this report. PPO Publication no. 568; at € 30,- The fourteenth workshop and proceedings were sponsored by the companies: BASF, Bayer CropScience, Belchim, Gowan, Indofil, Nufarm, DuPont, Syngenta, Makhteshim Agan, United Phosphorus, HZPC and ISK Bioscience. Applied Plant Research (Praktijkonderzoek Plant & Omgeving, PPO) part of Wageningen UR, is the ultimate knowledge institute for arable Farming, Multifunctional Agriculture and Field Production of Vegatables. Address: P.O. Box 430, NL-8200 AK Lelystad, The Netherlands Tel. -

Lihottaako Peruna?
Lihottaako peruna? Perunan ja pastan vertailu glykeemisen indeksin käsitteen valossa. Paavo Ahvenniemi, 22.12.2008 SISÄLLYSLUETTELO 1. Johdanto 2. Perunalla edullinen energiatase 3. Glykeeminen indeksi 4. Eri ruoka-aineiden glykeemiset indeksit 5. Glykeeminen kuorma 6. Keskiarvon harha 7. Yhteenveto Lähteet Liitteet 1. Johdanto Peruna on joutunut viime aikoina yhä kiihtyvämpänä käyvässä terveyskeskustelussa muiden tärkkelyspitoisten ruoka-aineiden tavoin tulilinjalle. Se onkin muka peruna joka nyt lihottaa, ja perusteena pidetään perunan, ja muiden tärkkelyspitoisten ruoka-aineiden, korkeaa niin kutsuttua glykeemistä indeksiä (GI). Glykeeminen indeksi kuvaa ravintoaineen tehokkuutta nostaa veren sokeripitoisuutta. Tämän selvityksen tarkoituksena on tutkia, onko perusteita pitää perunaa pastaa haitallisempana lihottavana ruoka-aineena. 2. Perunalla edullinen energiatase Kokonaisenergiataloudellisesti tarkasteltuna väite perunan lihottavuudesta on täysin naurettava. Käsitykseni perustuu siihen yksinkertaiseen tosiasiaan, että perunan energiatiheys on useihin muihin elintarvikkeisiin verrattuna hyvin matala (Taulukko 1). Sata grammaa keitettyä perunaa sisältää energiaa vain 63 kcal, kun esimerkiksi vastaavassa määrässä pastaa, joka pääsee lähimmäksi perunaa, on energiaa 95 kcal, ja aamiaishiutaleissa 364 kcal. Jos esimerkiksi henkistä työtä tekevä nainen täyttäisi koko päivittäisen keskimäärin 2200 kilokalorin energiantarpeen keitetyllä perunalla, hän saisi popsia sitä lähes 3.5 kiloa. Ekvivalentti pastamäärä olisi noin 2.3 kiloa, mutta -
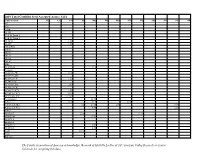
2019 Total Certified Seed Accepted Acres
2019 Total Certified Seed Accepted Acres - USA Cultivar/clone AK CA CO ID ME MI MN MT NE ND NV NY OR 387 6363 7 Four 7 05-152 07 H 56 Dorm 1 07 H 83 Dorm 1 08-181R 1.50 08-407 1.50 105/2/W06 318-33 503-22 504-35 907-15 902-47 Abby Ac Chaleur 12.8 Acoustic AC00206-2W 0.16 AC00395-2RU AC03433-1W 1.25 AC3452-2W AC05039-2RU 2.82 AC05153-1W AC05175-3P/Y 1.59 AC10376-1W/Y 0.19 AC99330-1 P/Y 19.87 Accumulator 84.76 Actrice 0.10 31.7 0.20 Adirondack Blue 0.2 21.78 2.00 0.78 Adirondack Red 4.44 0.31 Adora AF4124-7 25.6 13.19 AF4157-06 27.41 AF4172-2 AF4648-2 AF4659-12 6.02 AF5040-8 0.12 Agata 429.8 Aiata Albertine The Potato Association of America acknowledges the work of Michelle Leckler of CSU San Luis Valley Research in Center, Colorado for compiling this data. Cultivar/clone AK CA CO ID ME MI MN MT NE ND NV NY OR Alegria 65.00 266.10 19.00 2.95 Alexia All Blue 1.50 2.37 8 16.74 2.98 1.33 All Red Allagash 0.10 Allians Allora 3.2 Almera 17 Alpine Russet 51 57.20 9.9 Alta Cloud 0.10 Alturas 1085.33 622.92 29.65 1.80 83.00 Alverstone Russet 0.1 Amany Amarillo Moon Amarosa 7.00 1.7 0.54 7.86 Amey 0.71 Amisk Andover 72.34 14.27 Annabelle Anuschka 0.5 19.81 Anya Apache 19.00 AO012-5 AO06191-1 4.84 AO3141-6 1.05 AO3158-2TE A06021-1T (La Bella Russet) AO96305-3 AO96141-3 Arizona 1.12 25.37 0.18 Asterix 12.11 ATC00293-1W/Y Atlantic 155.76 44 913.75 16.00 41.86 18.02 141.52 226.22 82.34 ATTX-961014-1RY ATX0624s-4R/Y ATX91137-1RU Audrey 0.12 Austrian Crescent 9.87 48.5 3.15 1.01 24.00 2.70 Avalanche Axion B2727-2 0.5 Baby Boomer Cultivar/clone AK CA CO -

Maloney2020 Redacted.Pdf (5.995Mb)
This thesis has been submitted in fulfilment of the requirements for a postgraduate degree (e.g. PhD, MPhil, DClinPsychol) at the University of Edinburgh. Please note the following terms and conditions of use: This work is protected by copyright and other intellectual property rights, which are retained by the thesis author, unless otherwise stated. A copy can be downloaded for personal non-commercial research or study, without prior permission or charge. This thesis cannot be reproduced or quoted extensively from without first obtaining permission in writing from the author. The content must not be changed in any way or sold commercially in any format or medium without the formal permission of the author. When referring to this work, full bibliographic details including the author, title, awarding institution and date of the thesis must be given. A Decision Aid to Support the Use of Curative Late Blight Fungicides Kyran Maloney Doctor of Philosophy 2 2 ii Declaration This thesis has been authored by myself, and is substantially my own work. The work has not been previously submitted as part of any other degree of professional qualification. Kyran Maloney February, 2020 iii iv Table of Contents Acknowledgements.................................................................................................ix Abstract...................................................................................................................xi Lay summary........................................................................................................xiii