Alterations in Intestinal Microbiota Correlate with Susceptibility to Type 1 Diabetes
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Macellibacteroides Fermentans Gen. Nov., Sp. Nov., a Member of the Family Porphyromonadaceae Isolated from an Upflow Anaerobic Filter Treating Abattoir Wastewaters
International Journal of Systematic and Evolutionary Microbiology (2012), 62, 2522–2527 DOI 10.1099/ijs.0.032508-0 Macellibacteroides fermentans gen. nov., sp. nov., a member of the family Porphyromonadaceae isolated from an upflow anaerobic filter treating abattoir wastewaters Linda Jabari,1,2 Hana Gannoun,2 Jean-Luc Cayol,1 Abdeljabbar Hedi,1 Mitsuo Sakamoto,3 Enevold Falsen,4 Moriya Ohkuma,3 Moktar Hamdi,2 Guy Fauque,1 Bernard Ollivier1 and Marie-Laure Fardeau1 Correspondence 1Aix-Marseille Universite´ du Sud Toulon-Var, CNRS/INSU, IRD, MIO, UM 110, Case 925, Marie-Laure Fardeau 163 Avenue de Luminy, 13288 Marseille Cedex 9, France [email protected] 2Laboratoire d’Ecologie et de Technologie Microbienne, Institut National des Sciences Applique´es et de Technologie, Centre Urbain Nord, BP 676, 1080 Tunis Cedex, Tunisia 3Microbe Division/Japan Collection of Microorganisms, RIKEN BioResource Center 2-1 Hirosawa, Wako, Saitama 351-0198, Japan 4CCUG, Culture Collection, Department of Clinical Bacteriology, University of Go¨teborg, 41346 Go¨teborg, Sweden A novel obligately anaerobic, non-spore-forming, rod-shaped mesophilic bacterium, which stained Gram-positive but showed the typical cell wall structure of Gram-negative bacteria, was isolated from an upflow anaerobic filter treating abattoir wastewaters in Tunisia. The strain, designated LIND7HT, grew at 20–45 6C (optimum 35–40 6C) and at pH 5.0–8.5 (optimum pH 6.5–7.5). It did not require NaCl for growth, but was able to grow in the presence of up to 2 % NaCl. Sulfate, thiosulfate, elemental sulfur, sulfite, nitrate and nitrite were not used as terminal electron acceptors. -

Comparative Genomics of the Genus Porphyromonas Identifies Adaptations for Heme Synthesis Within the Prevalent Canine Oral Species Porphyromonas Cangingivalis
GBE Comparative Genomics of the Genus Porphyromonas Identifies Adaptations for Heme Synthesis within the Prevalent Canine Oral Species Porphyromonas cangingivalis Ciaran O’Flynn1,*, Oliver Deusch1, Aaron E. Darling2, Jonathan A. Eisen3,4,5, Corrin Wallis1,IanJ.Davis1,and Stephen J. Harris1 1 The WALTHAM Centre for Pet Nutrition, Waltham-on-the-Wolds, United Kingdom Downloaded from 2The ithree Institute, University of Technology Sydney, Ultimo, New South Wales, Australia 3Department of Evolution and Ecology, University of California, Davis 4Department of Medical Microbiology and Immunology, University of California, Davis 5UC Davis Genome Center, University of California, Davis http://gbe.oxfordjournals.org/ *Corresponding author: E-mail: ciaran.ofl[email protected]. Accepted: November 6, 2015 Abstract Porphyromonads play an important role in human periodontal disease and recently have been shown to be highly prevalent in canine mouths. Porphyromonas cangingivalis is the most prevalent canine oral bacterial species in both plaque from healthy gingiva and at University of Technology, Sydney on January 17, 2016 plaque from dogs with early periodontitis. The ability of P. cangingivalis to flourish in the different environmental conditions char- acterized by these two states suggests a degree of metabolic flexibility. To characterize the genes responsible for this, the genomes of 32 isolates (including 18 newly sequenced and assembled) from 18 Porphyromonad species from dogs, humans, and other mammals were compared. Phylogenetic trees inferred using core genes largely matched previous findings; however, comparative genomic analysis identified several genes and pathways relating to heme synthesis that were present in P. cangingivalis but not in other Porphyromonads. Porphyromonas cangingivalis has a complete protoporphyrin IX synthesis pathway potentially allowing it to syn- thesize its own heme unlike pathogenic Porphyromonads such as Porphyromonas gingivalis that acquire heme predominantly from blood. -

Xiexin Tang Improves the Symptom of Type 2 Diabetic Rats by Modulation of the Gut Microbiota
www.nature.com/scientificreports OPEN Xiexin Tang improves the symptom of type 2 diabetic rats by modulation of the gut microbiota Received: 30 August 2017 Xiaoyan Wei, Jinhua Tao , Suwei Xiao, Shu Jiang, Erxin Shang, Zhenhua Zhu, Dawei Qian Accepted: 13 February 2018 & Jinao Duan Published: xx xx xxxx Type 2 diabetes mellitus (T2DM), a chronic metabolic disease which severely impairs peoples’ quality of life, currently attracted worldwide concerns. There are growing evidences that gut microbiota can exert a great impact on the development of T2DM. Xiexin Tang (XXT), a traditional Chinese medicine prescription, has been clinically used to treat diabetes for thousands of years. However, few researches are investigated on the modulation of gut microbiota community by XXT which will be very helpful to unravel how it works. In this study, bacterial communities were analyzed based on high-throughput 16S rRNA gene sequencing. Results indicated that XXT could notably shape the gut microbiota. T2DM rats treated with XXT exhibited obvious changes in the composition of the gut microbiota, especially for some short chain fatty acids producing and anti-infammatory bacteria such as Adlercreutzia, Alloprevotella, Barnesiella, [Eubacterium] Ventriosum group, Blautia, Lachnospiraceae UCG-001, Papillibacter and Prevotellaceae NK3B31 group. Additionally, XXT could also signifcantly ameliorate hyperglycemia, lipid metabolism dysfunction and infammation in T2DM rats. Moreover, the correlation analysis illustrated that the key microbiota had a close relationship with the T2DM related indexes. The results probably provided useful information for further investigation on its active mechanism and clinical application. T2DM, a chronic metabolic disease characterized by hyperglycemia as a result of insufcient insulin secretion, insulin action or both1, is estimated that its numbers in the adults will increase by 55% by 20352. -

Development of the Equine Hindgut Microbiome in Semi-Feral and Domestic Conventionally-Managed Foals Meredith K
Tavenner et al. Animal Microbiome (2020) 2:43 Animal Microbiome https://doi.org/10.1186/s42523-020-00060-6 RESEARCH ARTICLE Open Access Development of the equine hindgut microbiome in semi-feral and domestic conventionally-managed foals Meredith K. Tavenner1, Sue M. McDonnell2 and Amy S. Biddle1* Abstract Background: Early development of the gut microbiome is an essential part of neonate health in animals. It is unclear whether the acquisition of gut microbes is different between domesticated animals and their wild counterparts. In this study, fecal samples from ten domestic conventionally managed (DCM) Standardbred and ten semi-feral managed (SFM) Shetland-type pony foals and dams were compared using 16S rRNA sequencing to identify differences in the development of the foal hindgut microbiome related to time and management. Results: Gut microbiome diversity of dams was lower than foals overall and within groups, and foals from both groups at Week 1 had less diverse gut microbiomes than subsequent weeks. The core microbiomes of SFM dams and foals had more taxa overall, and greater numbers of taxa within species groups when compared to DCM dams and foals. The gut microbiomes of SFM foals demonstrated enhanced diversity of key groups: Verrucomicrobia (RFP12), Ruminococcaceae, Fusobacterium spp., and Bacteroides spp., based on age and management. Lactic acid bacteria Lactobacillus spp. and other Lactobacillaceae genera were enriched only in DCM foals, specifically during their second and third week of life. Predicted microbiome functions estimated computationally suggested that SFM foals had higher mean sequence counts for taxa contributing to the digestion of lipids, simple and complex carbohydrates, and protein. -

Gut Microbiome Composition Remains Stable in Individuals with Diabetes-Related Early to Late Stage Chronic Kidney Disease
biomedicines Article Gut Microbiome Composition Remains Stable in Individuals with Diabetes-Related Early to Late Stage Chronic Kidney Disease Ashani Lecamwasam 1,2,3,*, Tiffanie M. Nelson 4, Leni Rivera 3, Elif I. Ekinci 2,5, Richard Saffery 1,6 and Karen M. Dwyer 3 1 Epigenetics Research, Murdoch Children’s Research Institute, VIC 3052, Australia; [email protected] 2 Department of Endocrinology, Austin Health, VIC 3079, Australia; [email protected] 3 School of Medicine, Faculty of Health, Deakin University, VIC 3220, Australia; [email protected] (L.R.); [email protected] (K.M.D.) 4 Menzies Health Institute Queensland, Griffith University, QLD 4222, Australia; [email protected] 5 Department of Medicine, University of Melbourne, VIC 3010, Australia 6 Department of Paediatrics, University of Melbourne, VIC 3010, Australia * Correspondence: [email protected]; Tel.: +613-8341-6200; Fax: +613-9348-1391 Abstract: (1) Background: Individuals with diabetes and chronic kidney disease display gut dysbiosis when compared to healthy controls. However, it is unknown whether there is a change in dysbiosis across the stages of diabetic chronic kidney disease. We investigated a cross-sectional study of patients with early and late diabetes associated chronic kidney disease to identify possible microbial differences between these two groups and across each of the stages of diabetic chronic kidney disease. (2) Methods: This cross-sectional study recruited 95 adults. DNA extracted from collected stool samples were used for 16S rRNA sequencing to identify the bacterial community in the Citation: Lecamwasam, A.; Nelson, gut. (3) Results: The phylum Firmicutes was the most abundant and its mean relative abundance T.M.; Rivera, L.; Ekinci, E.I.; Saffery, was similar in the early and late chronic kidney disease group, 45.99 ± 0.58% and 49.39 ± 0.55%, R.; Dwyer, K.M. -
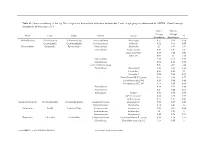
Genus Contributing to the Top 70% of Significant Dissimilarity of Bacteria Between Day 7 and 18 Age Groups As Determined by SIMPER
Table S1: Genus contributing to the top 70% of significant dissimilarity of bacteria between day 7 and 18 age groups as determined by SIMPER. Overall average dissimilarity between ages is 51%. Day 7 Day 18 Average Average Phyla Class Order Family Genus % Abundance Abundance Actinobacteria Actinobacteria Actinomycetales Actinomycetaceae Actinomyces 0.57 0.24 0.74 Coriobacteriia Coriobacteriales Coriobacteriaceae Collinsella 0.51 0.61 0.57 Bacteroidetes Bacteroidia Bacteroidales Bacteroidaceae Bacteroides 2.1 1.63 1.03 Marinifilaceae Butyricimonas 0.82 0.81 0.9 Sanguibacteroides 0.08 0.42 0.59 CAG-873 0.01 1.1 1.68 Marinifilaceae 0.38 0.78 0.91 Marinifilaceae 0.78 1.04 0.97 p-2534-18B5 gut group 0.06 0.7 1.04 Prevotellaceae Alloprevotella 0.46 0.83 0.89 Prevotella 2 0.95 1.11 1.3 Prevotella 7 0.22 0.42 0.56 Prevotellaceae NK3B31 group 0.62 0.68 0.77 Prevotellaceae UCG-003 0.26 0.64 0.89 Prevotellaceae UCG-004 0.11 0.48 0.68 Prevotellaceae 0.64 0.52 0.89 Prevotellaceae 0.27 0.42 0.55 Rikenellaceae Alistipes 0.39 0.62 0.77 dgA-11 gut group 0.04 0.38 0.56 RC9 gut group 0.79 1.17 0.95 Epsilonbacteraeota Campylobacteria Campylobacterales Campylobacteraceae Campylobacter 0.58 0.83 0.97 Helicobacteraceae Helicobacter 0.12 0.41 0.6 Firmicutes Bacilli Lactobacillales Enterococcaceae Enterococcus 0.36 0.31 0.64 Lactobacillaceae Lactobacillus 1.4 1.24 1 Streptococcaceae Streptococcus 0.82 0.58 0.53 Firmicutes Clostridia Clostridiales Christensenellaceae Christensenellaceae R-7 group 0.38 1.36 1.56 Clostridiaceae Clostridium sensu stricto 1 1.16 0.58 -

Role of Actinobacteria and Coriobacteriia in the Antidepressant Effects of Ketamine in an Inflammation Model of Depression
Pharmacology, Biochemistry and Behavior 176 (2019) 93–100 Contents lists available at ScienceDirect Pharmacology, Biochemistry and Behavior journal homepage: www.elsevier.com/locate/pharmbiochembeh Role of Actinobacteria and Coriobacteriia in the antidepressant effects of ketamine in an inflammation model of depression T Niannian Huanga,1, Dongyu Huaa,1, Gaofeng Zhana, Shan Lia, Bin Zhub, Riyue Jiangb, Ling Yangb, ⁎ ⁎ Jiangjiang Bia, Hui Xua, Kenji Hashimotoc, Ailin Luoa, , Chun Yanga, a Department of Anesthesiology, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan 430030, China b Department of Internal Medicine, The Third Affiliated Hospital of Soochow University, Changzhou 213003, China c Division of Clinical Neuroscience, Chiba University Center for Forensic Mental Health, Chiba 260-8670, Japan ARTICLE INFO ABSTRACT Keywords: Ketamine, an N-methyl-D-aspartic acid receptor (NMDAR) antagonist, elicits rapid-acting and sustained anti- Ketamine depressant effects in treatment-resistant depressed patients. Accumulating evidence suggests that gut microbiota Depression via the gut-brain axis play a role in the pathogenesis of depression, thereby contributing to the antidepressant Lipopolysaccharide actions of certain compounds. Here we investigated the role of gut microbiota in the antidepressant effects of Gut microbiota ketamine in lipopolysaccharide (LPS)-induced inflammation model of depression. Ketamine (10 mg/kg) sig- nificantly attenuated the increased immobility time in forced swimming test (FST), which was associated with the improvements in α-diversity, consisting of Shannon, Simpson and Chao 1 indices. In addition to α-diversity, β-diversity, such as principal coordinates analysis (PCoA), and linear discriminant analysis (LDA) coupled with effect size measurements (LEfSe), showed a differential profile after ketamine treatment. -

Annotations of the Otus with the Lowest P-Values for SR Samples at 0 and 4H Fermentation Time Points
Electronic Supplementary Material (ESI) for Food & Function. This journal is © The Royal Society of Chemistry 2016 Table S1. Taxonomic annotations: Annotations of the OTUs with the lowest p-values for SR samples at 0 and 4h fermentation time points. TAXA ID PHYLUM CLASS ORDER FAMILY GENUS SPECIES 4452633 Firmicutes Clostridia Clostridiales Clostridiaceae unclassified unclassified 191399 Firmicutes Clostridia Clostridiales Ruminococcaceae unclassified unclassified 4256470 Bacteroidetes Bacteroidia Bacteroidales Bacteroidaceae Bacteroides 97otu97833 179291 Firmicutes Clostridia Clostridiales Ruminococcaceae Faecalibacterium prausnitzii 192046 Firmicutes Clostridia Clostridiales unclassified unclassified unclassified 4315974 Firmicutes Bacilli Lactobacillales Streptococcaceae Streptococcus 97otu66887 4343627 Bacteroidetes Bacteroidia Bacteroidales Bacteroidaceae Bacteroides fragilis 760967 Bacteroidetes Bacteroidia Bacteroidales Prevotellaceae Prevotella 97otu12794 4475758 Firmicutes Clostridia Clostridiales Veillonellaceae Veillonella unclassified 566976 Bacteroidetes Gammapro Bacteroidales Bacteroidaceae Bacteroides unclassified teobacteria 328617 Bacteroidetes Bacteroidia Bacteroidales Bacteroidaceae Bacteroides uniformis 16054 Firmicutes Clostridia Clostridiales Ruminococcaceae Ruminococcus 97otu97520 SR: sun-dried raisins. Table S2. Taxonomic annotations: Annotations of the OTUs with the lowest p-values for SR samples at 0 and 8h fermentation time points. TAXA ID PHYLUM CLASS ORDER FAMILY GENUS SPECIES 194443 Firmicutes Clostridia Clostridiales -

Gut Microbiota Differs in Composition and Functionality Between Children
Diabetes Care Volume 41, November 2018 2385 Gut Microbiota Differs in Isabel Leiva-Gea,1 Lidia Sanchez-Alcoholado,´ 2 Composition and Functionality Beatriz Mart´ın-Tejedor,1 Daniel Castellano-Castillo,2,3 Between Children With Type 1 Isabel Moreno-Indias,2,3 Antonio Urda-Cardona,1 Diabetes and MODY2 and Healthy Francisco J. Tinahones,2,3 Jose´ Carlos Fernandez-Garc´ ´ıa,2,3 and Control Subjects: A Case-Control Mar´ıa Isabel Queipo-Ortuno~ 2,3 Study Diabetes Care 2018;41:2385–2395 | https://doi.org/10.2337/dc18-0253 OBJECTIVE Type 1 diabetes is associated with compositional differences in gut microbiota. To date, no microbiome studies have been performed in maturity-onset diabetes of the young 2 (MODY2), a monogenic cause of diabetes. Gut microbiota of type 1 diabetes, MODY2, and healthy control subjects was compared. PATHOPHYSIOLOGY/COMPLICATIONS RESEARCH DESIGN AND METHODS This was a case-control study in 15 children with type 1 diabetes, 15 children with MODY2, and 13 healthy children. Metabolic control and potential factors mod- ifying gut microbiota were controlled. Microbiome composition was determined by 16S rRNA pyrosequencing. 1Pediatric Endocrinology, Hospital Materno- Infantil, Malaga,´ Spain RESULTS 2Clinical Management Unit of Endocrinology and Compared with healthy control subjects, type 1 diabetes was associated with a Nutrition, Laboratory of the Biomedical Research significantly lower microbiota diversity, a significantly higher relative abundance of Institute of Malaga,´ Virgen de la Victoria Uni- Bacteroides Ruminococcus Veillonella Blautia Streptococcus versityHospital,Universidad de Malaga,M´ alaga,´ , , , , and genera, and a Spain lower relative abundance of Bifidobacterium, Roseburia, Faecalibacterium, and 3Centro de Investigacion´ BiomedicaenRed(CIBER)´ Lachnospira. -

Description of Gabonibacter Massiliensis Gen. Nov., Sp. Nov., a New Member of the Family Porphyromonadaceae Isolated from the Human Gut Microbiota
Curr Microbiol DOI 10.1007/s00284-016-1137-2 Description of Gabonibacter massiliensis gen. nov., sp. nov., a New Member of the Family Porphyromonadaceae Isolated from the Human Gut Microbiota 1,2 1 3,4 Gae¨l Mourembou • Jaishriram Rathored • Jean Bernard Lekana-Douki • 5 1 1 Ange´lique Ndjoyi-Mbiguino • Saber Khelaifia • Catherine Robert • 1 1,6 1 Nicholas Armstrong • Didier Raoult • Pierre-Edouard Fournier Received: 9 June 2016 / Accepted: 8 September 2016 Ó Springer Science+Business Media New York 2016 Abstract The identification of human-associated bacteria Gabonibacter gen. nov. and the new species G. mas- is very important to control infectious diseases. In recent siliensis gen. nov., sp. nov. years, we diversified culture conditions in a strategy named culturomics, and isolated more than 100 new bacterial Keywords Gabonibacter massiliensis Á Taxonogenomics Á species and/or genera. Using this strategy, strain GM7, a Culturomics Á Gabon Á Gut microbiota strictly anaerobic gram-negative bacterium was recently isolated from a stool specimen of a healthy Gabonese Abbreviations patient. It is a motile coccobacillus without catalase and CSUR Collection de Souches de l’Unite´ des oxidase activities. The genome of Gabonibacter mas- Rickettsies siliensis is 3,397,022 bp long with 2880 ORFs and a G?C DSM Deutsche Sammlung von content of 42.09 %. Of the predicted genes, 2,819 are Mikroorganismen protein-coding genes, and 61 are RNAs. Strain GM7 differs MALDI-TOF Matrix-assisted laser desorption/ from the closest genera within the family Porphyromon- MS ionization time-of-flight mass adaceae both genotypically and in shape and motility. -

Bacteroides Gingivalis, and Bacteroides Endodontalis in a New Genus, Porphyromonas H
INTERNATIONALJOURNAL OF SYSTEMATICBACTERIOLOGY, Jan. 1988, p. 128-131 Vol. 38, No. 1 0020-7713/88/010128-04$02.00/0 Copyright 0 1988, International Union of Microbiological Societies Proposal for Reclassification of Bacteroides asaccharolyticus , Bacteroides gingivalis, and Bacteroides endodontalis in a New Genus, Porphyromonas H. N. SHAH1* AND M. D. COLLINS’ Department of Oral Microbiology, London Hospital Medical College, London El 2AD,l and Division of Microbiology, AFRC Institute of Food Research, Shinfeld, Reading RG2 9AT,= United Kingdom The asaccharolytic, pigmented Bacteroides, Bacteroides asaccharolyticus, Bacteroides gingivalis, and Bacte- roides endodontalis, form a group of relatively homogeneous species which differ markedly in biochemical and chemical properties from the type species of Bacteroides, Bacteroides fragilis (Castellani and Chalmers), such that they should not be retained within this genus. Therefore, we propose that Bacteroides asaccharolyticus (Holdeman and Moore) Finegold and Barnes, Bacteroides gingivalis Coykendhll, Kaczmarek and Slots, and Bacteroides endodontalis van Steenbergen, van Winkelhoff, Mayrand, Grenier and de Graaff be reclassified in a new genus, Porphyromonas, as Porphyromonas asaccharolytica comb. nov., Porphyromonas gingivalis comb. nov., and Porphyromonas endodontalis comb. nov., respectively. In Bergey ’s Manual of Determinative Bacteriology, 8th quite unrelated to the type species of the genus Bacteroides, ed. (12), the asaccharolytic, pigmented Bacteroides were Bacteroides fragilis. All of the asaccharolytic, pigmented regarded as a single homogeneous taxon, Bacteroides mela- bacteroides accumulate major levels of protoheme rather ninogenicus subsp. asaccharolyticus. As the clinical signif- than protoporphyrin when cells are cultured on blood agar icance of these microorganisms in oral cavities was recog- (3,25, 27). The three species are nonfermentative and utilize nized, extensive taxonomic studies were carried out. -

Endosymbiont Diversity and Evolution Across Weevil Tree of Life
bioRxiv preprint doi: https://doi.org/10.1101/171181; this version posted August 1, 2017. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC 4.0 International license. Endosymbiont diversity and evolution across weevil tree of life Guanyang Zhang1#, Patrick Browne1,2#, Geng Zhen1#, Andrew Johnston4, Hinsby Cadillo-Quiroz5, and Nico Franz1 1 School of Life Sciences, Arizona State University, Tempe, Arizona, USA 2 Department of Environmental Science, Aarhus University, 4000 Roskilde, Denmark # These authors contributed equally to this work ABSTRACT As early as the time of Paul Buchner, a pioneer of endosymbionts research, it was shown that weevils host diverse bacterial endosymbionts, probably only second to the hemipteran insects. To date, there is no taxonomically broad survey of endosymbionts in weevils, which preclude any systematic understanding of the diversity and evolution of endosymbionts in this large group of insects, which comprise nearly 7% of described diversity of all insects. We gathered the largest known taxonomic sample of weevils representing four families and 17 subfamilies to perform a study of weevil endosymbionts. We found that the diversity of endosymbionts is exceedingly high, with as many as 44 distinct kinds of endosymbionts detected. We recovered an ancient origin of association of Nardonella with weevils, dating back to 124 MYA. We found repeated losses of this endosymbionts, but also cophylogeny with weevils. We also investigated patterns of coexistence and coexclusion. INTRODUCTION Weevils (Insecta: Coleoptera: Curculionoidea) host diverse bacterial endosymbionts.