Gallagher, Alan.Pdf
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Anet Newsletter 8
30 APRIL 2006 No. 8 ANeT Newsletter International Network for the Study of Asian Ants / DIWPA Network for Social Insect Collections / DIVERSITAS in West Pacific and Asia Proceedings of Committee Meeting of 5th ANeT Workshop Minutes prepared by: Prof. Datin Dr. Maryati Mohamed Institute for Tropical Biology & Conservation Universiti Malaysia Sabah, MALAYSIA Place and Date of the Committee Meeting Committee meeting of 5th ANeT Workshop was held on 30th November 2005 at the National Museum, Kuala Lumpur. The meeting started at 12.30 with a discussion on the draft of Action Plan tabled by Dr. John Fellowes and meeting then chaired by Prof. Maryati Mohamed at 1.00 pm. Meeting adjourned at 3.00 p.m. Members Attending Prof. Maryati Mohamed, the President of ANeT (Malaysia) Prof. Seiki Yamane (Japan) Prof. Kazuo Ogata (Japan) Dr. Rudy Kohout (Australia) Dr. John R. Fellowes (Hong Kong/UK) Mr. Suputa (Indonesia) Dr. Yoshiaki Hashimoto (Japan) Dr. Decha Wiwatwitaya (Thailand) Dr. Bui Tuan Viet (Vietnam) Dr. Himender Bharti (India) Dr. Sriyani Dias (Sri Lanka) Mr. Bakhtiar Effendi Yahya, the Secretariat of ANeT (Japan) Ms. Petherine Jimbau, the Secretariat of ANeT (Malaysia) Agenda Agreed 1. Discussion on Proposal on Action Plan as tabled by Dr. John Fellowes 2. Proceedings/Journal 3. Next meeting - 6th ANeT Seminar and Meeting (date and venue) 4. New members and structure of committee membership 5. Any other business ANeT Newsletter No. 8. 30 April 2006 Agenda Item 1: Discussion on Proposal on Action Plan as tabled by Dr. John Fellowes Draft of Proposal was distributed. During the discussion no amendments were proposed to the draft Action Plan objectives. -

Larvae of the Green Lacewing Mallada Desjardinsi (Neuroptera: Chrysopidae) Protect Themselves Against Aphid-Tending Ants by Carrying Dead Aphids on Their Backs
Appl Entomol Zool (2011) 46:407–413 DOI 10.1007/s13355-011-0053-y ORIGINAL RESEARCH PAPER Larvae of the green lacewing Mallada desjardinsi (Neuroptera: Chrysopidae) protect themselves against aphid-tending ants by carrying dead aphids on their backs Masayuki Hayashi • Masashi Nomura Received: 6 March 2011 / Accepted: 11 May 2011 / Published online: 28 May 2011 Ó The Japanese Society of Applied Entomology and Zoology 2011 Abstract Larvae of the green lacewing Mallada desj- Introduction ardinsi Navas are known to place dead aphids on their backs. To clarify the protective role of the carried dead Many ants tend myrmecophilous homopterans such as aphids against ants and the advantages of carrying them for aphids and scale insects, and utilize the secreted honeydew lacewing larvae on ant-tended aphid colonies, we carried as a sugar resource; in return, the homopterans receive out some laboratory experiments. In experiments that beneficial services from the tending ants (Way 1963; Breton exposed lacewing larvae to ants, approximately 40% of the and Addicott 1992; Nielsen et al. 2010). These mutualistic larvae without dead aphids were killed by ants, whereas no interactions between ants and homopterans reduce the larvae carrying dead aphids were killed. The presence of survival and abundance of other arthropods, including the dead aphids did not affect the attack frequency of the non-honeydew-producing herbivores and other predators ants. When we introduced the lacewing larvae onto plants (Bristow 1984; Buckley 1987; Suzuki et al. 2004; Kaplan colonized by ant-tended aphids, larvae with dead aphids and Eubanks 2005), because the tending ants become more stayed for longer on the plants and preyed on more aphids aggressive and attack arthropods that they encounter on than larvae without dead aphids. -

Local Nature Reserve Management Plan 2020 – 2024
Bisley Road Cemetery, Stroud Local Nature Reserve Management Plan 2020 – 2024 Prepared for Stroud Town Council CONTENTS 1 VISION STATEMENT 2 POLICY STATEMENTS 3 GENERAL DESCRIPTION 3.1 General background information 3.1.1 Location and site boundaries Map 1 Site Location 3.1.2 Tenure Map 2 Schedule Plan 3.1.3 Management/organisational infrastructure 3.1.4 Site infrastructure 3.1.5 Map coverage 3.2 Environmental information 3.2.1 Physical 3.2.2 Biological 3.2.2.1 Habitats Map 3 Compartment Map – Old Cemetery Map 4 Compartment Map – New Cemetery 3.2.2.2 Flora 3.2.2.3 Fauna 3.3 Cultural 3.3.1 Past land use 3.3.2 Present land use 3.3.3 Past management for nature conservation 3.3.4 Present legal status 4 NATURE CONSERVATION FEATURES OF INTEREST 4.1 Identification and confirmation of conservation features 4.2 Objectives 4.2.1 Unimproved grassland 4.2.1.1 Summary description 4.2.1.2 Management objectives 4.2.1.3 Performance indicators 4.2.1.4 Conservation status 4.2.1.5 Rationale 4.2.1.6 Management projects 4.2.2 Trees and Woodland 4.2.2.1 Summary description 4.2.2.2 Management objectives 4.2.2.3 Performance indicators 4.2.2.4 Conservation status 4.2.2.5 Rationale 4.2.2.6 Management projects 4.2.3 Lichens 4.2.3.1 Summary description 4.2.3.2 Management objectives 4.2.3.3 Performance indicators 4.2.3.4 Conservation status 4.2.3.5 Rationale 4.2.3.6 Management projects 4.3 Rationale & Proposals per compartment Bisley Rd Cemetery Mgmt Plan 2020-2024 2 5 HISTORIC INTEREST 5.1 Confirmation of conservation features 5.2 Objectives 5.3 Rationale 6 STAKEHOLDERS 6.1 Evaluation 6.2 Management projects 7 ACCESS / TOURISM 7.1 Evaluation 7.2 Management objectives 8 INTERPRETATION 8.1 Evaluation 8.2 Management Projects 9 OPERATIONAL OBJECTIVES 9.1 Operational objectives 9.2 Management projects 10 WORK PLAN Appendix 1 Species List Bisley Rd Cemetery Mgmt Plan 2020-2024 3 1 VISION STATEMENT Stroud Town Council are committed to conserving Stroud Cemetery to: • Enable the people of Stroud to always have a place of peace and quiet reflection and recreation. -

Folk Taxonomy, Nomenclature, Medicinal and Other Uses, Folklore, and Nature Conservation Viktor Ulicsni1* , Ingvar Svanberg2 and Zsolt Molnár3
Ulicsni et al. Journal of Ethnobiology and Ethnomedicine (2016) 12:47 DOI 10.1186/s13002-016-0118-7 RESEARCH Open Access Folk knowledge of invertebrates in Central Europe - folk taxonomy, nomenclature, medicinal and other uses, folklore, and nature conservation Viktor Ulicsni1* , Ingvar Svanberg2 and Zsolt Molnár3 Abstract Background: There is scarce information about European folk knowledge of wild invertebrate fauna. We have documented such folk knowledge in three regions, in Romania, Slovakia and Croatia. We provide a list of folk taxa, and discuss folk biological classification and nomenclature, salient features, uses, related proverbs and sayings, and conservation. Methods: We collected data among Hungarian-speaking people practising small-scale, traditional agriculture. We studied “all” invertebrate species (species groups) potentially occurring in the vicinity of the settlements. We used photos, held semi-structured interviews, and conducted picture sorting. Results: We documented 208 invertebrate folk taxa. Many species were known which have, to our knowledge, no economic significance. 36 % of the species were known to at least half of the informants. Knowledge reliability was high, although informants were sometimes prone to exaggeration. 93 % of folk taxa had their own individual names, and 90 % of the taxa were embedded in the folk taxonomy. Twenty four species were of direct use to humans (4 medicinal, 5 consumed, 11 as bait, 2 as playthings). Completely new was the discovery that the honey stomachs of black-coloured carpenter bees (Xylocopa violacea, X. valga)were consumed. 30 taxa were associated with a proverb or used for weather forecasting, or predicting harvests. Conscious ideas about conserving invertebrates only occurred with a few taxa, but informants would generally refrain from harming firebugs (Pyrrhocoris apterus), field crickets (Gryllus campestris) and most butterflies. -

The Role of Ant Nests in European Ground Squirrel's (Spermophilus
Biodiversity Data Journal 7: e38292 doi: 10.3897/BDJ.7.e38292 Short Communications The role of ant nests in European ground squirrel’s (Spermophilus citellus) post-reintroduction adaptation in two Bulgarian mountains Maria Kachamakova‡‡, Vera Antonova , Yordan Koshev‡ ‡ Institute of Biodiversity and Ecosystem Research at Bulgarian Academy of Sciences, Sofia, Bulgaria Corresponding author: Maria Kachamakova ([email protected]) Academic editor: Ricardo Moratelli Received: 16 Jul 2019 | Accepted: 09 Sep 2019 | Published: 07 Oct 2019 Citation: Kachamakova M, Antonova V, Koshev Y (2019) The role of ant nests in European ground squirrel’s (Spermophilus citellus) post-reintroduction adaptation in two Bulgarian mountains. Biodiversity Data Journal 7: e38292. https://doi.org/10.3897/BDJ.7.e38292 Abstract The European ground squirrel (Spermophilus citellus) is a vulnerable species, whose populations are declining throughout its entire range in Central and South-Eastern Europe. To a great extent, its conservation depends on habitat restoration, maintenance and protection. In order to improve the conservation status of the species, reintroductions are increasingly applied. Therefore, researchers focus their attention on factors that facilitate these activities and contribute to their success. In addition to the well-known factors like grass height and exposition, others, related to the underground characteristics, are more difficult to evaluate. The presence of other digging species could help this evaluation. Here, we present two reintroduced ground squirrel colonies, where the vast majority of the burrows are located in the base of anthills, mainly of yellow meadow ant (Lasius flavus). This interspecies relationship offers numerous advantages for the ground squirrel and is mostly neutral for the ants. -
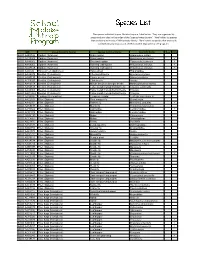
Species List
The species collected in your Malaise trap are listed below. They are organized by group and are listed in the order of the 'Species Image Library'. ‘New’ refers to species that are brand new to our DNA barcode library. 'Rare' refers to species that were only collected in your trap out of all 67 that were deployed for the program. BIN Group (Scientific Name) Species Common Name Scientific Name New Rare BOLD:AAB5726 Spiders (Araneae) Grass spider Agelenopsis potteri BOLD:AAD6926 Spiders (Araneae) Ghost spider Anyphaena pectorosa BOLD:AAH0002 Spiders (Araneae) Sheetweb spider Tapinocyba hortensis BOLD:AAN9005 Spiders (Araneae) Running crab spider Philodromus minutus BOLD:ACX7149 Spiders (Araneae) Running crab spider Philodromus minutus BOLD:AAI4346 Harvestmen (Opiliones) Harvestman Phalangiidae BOLD:AAU6970 Beetles (Coleoptera) Checkered beetle Enoclerus nigripes BOLD:AAH0130 Beetles (Coleoptera) Clover weevil Sitona hispidulus BOLD:ACP9027 Beetles (Coleoptera) Click beetle Aeolus BOLD:AAN6154 Beetles (Coleoptera) Minute brown scavenger beetle Melanophthalma inermis BOLD:AAN6148 Beetles (Coleoptera) False metallic wood-boring beetle Trixagus carinicollis BOLD:AAU6966 Beetles (Coleoptera) False metallic wood-boring beetle Trixagus BOLD:ABW2869 Beetles (Coleoptera) False metallic wood-boring beetle Trixagus BOLD:AAG9897 Earwigs (Dermaptera) European earwig Forficula auricularia-A BOLD:AAG2513 Flies (Diptera) Root maggot fly Eustalomyia BOLD:AAH2351 Flies (Diptera) Robber fly Machimus sadyates BOLD:AAN5137 Flies (Diptera) March fly Penthetria -

Book of Abstracts Goettingen O
Imprint Geschäftstelle der Ge‐ Verhandlungen der Gesellschaft sellschaft für Ökologie für Ökologie, Band 45 Institut für Ökologie Herausgegeben im Auftrag der Gesell‐ Technische Universität schaft für Ökologie von apl. Prof. Dr. Berlin Rothenburgerstr. Michael Bredemeier, Sektion Waldöko‐ 12 systemforschung, Zentrum für Biodiver‐ D‐12165 Berlin sität und nachhaltige Landnutzung (CBL), Tel.: 030‐ Georg‐August‐Universität Göttingen 31471396 Fax.: 030‐31471355 © Gesellschaft für Ökologie Göttingen 2015, ISSN: 0171‐1113 Contact: KCS Kuhlmann Convention Service Universität Göttingen Heike Kuhmann Prof. Dr. Michael Bredemeier, Rue des Chênes Sektion Waldökosystemfor‐ 12 CH‐2800 De‐ schung, Büsgenweg 2 lémont D‐ 37077 Göttingen + 41‐32‐4234384 info@kcs‐convention.com The 45th annual conference of the Ecological Society of Germany, Austria and Switzer‐ land (GfÖ) is taking place from 31st August to 04th September 2015 at the Universi‐ ty of Göttingen. Host of the conference is the Centre for Biodiversity and Sustainable Land Use (CBL), University of Göttingen. Local Organizing Committee Chair: Prof. Dr. Christian Ammer, University of Göt‐ tingen Co‐Chairs: Prof. Dr. Teja Tscharntke, Prof. Dr. Kerstin Wie‐ gand, University of Göttingen Proceedings editors: Prof. Dr. Michael Bredemeier, Dr. Juliane Steckel, Ursula Seele, University of Göttingen Field Trip planning: Dr. Simone Pfeiffer, University of Göttingen Scientific Programm Committee Prof. Dr. Christian Ammer, Prof. Dr. Michael Bredemeier, Prof. Dr. Alexander Knohl, Prof. Dr. Holger Kreft, Dr. Simone Pfeiffer, PD Dr. Martin Potthoff, Prof. Dr. Stefan Scheu, Prof. Dr. Teja Tscharntke Dr. G. Wiedey, Prof. Dr. Kerstin Wiegand The book is also available for download as electronic document on the conference web site (www. gfoe‐2015.de) Production: Centre for Biodiversity and Sustainable Land Use (CBL), University of Göttingen, Büsgenweg 2, 37077 Göttingen, Germany Editors: Prof. -

Larvae of Two Ladybirds, Phymatosternus Lewisii And
Appl. Entomol. Zool. 42 (2): 181–187 (2007) http://odokon.org/ Larvae of two ladybirds, Phymatosternus lewisii and Scymnus posticalis (Coleoptera: Coccinellidae), exploiting colonies of the brown citrus aphid Toxoptera citricidus (Homoptera: Aphididae) attended by the ant Pristomyrmex pungens (Hymenoptera: Formicidae) Shuji KANEKO*,† Shizuoka Prefectural Citrus Experiment Station; Shimizu, Shizuoka 424–0905, Japan (Received 12 June 2006; Accepted 14 November 2006) Abstract The distribution of two small coccinellids, Phymatosternus lewisii and Scymnus posticalis, across colonies of the aphid Toxoptera citricidus in relation to ant-attendance of the colonies and ant species, behavioral interactions be- tween the coccinellid larvae and ants, and the overlap in the larval distribution of the two coccinellids were examined in a citrus grove in Japan. P. lewisii larvae were found frequently in aphid colonies attended by the ant Pristomyrmex pungens but rarely in colonies attended by another ant, Lasius japonicus, and in ant-excluded colonies. A number of S. posticalis larvae were also recorded in P. pungens-attended colonies and some larvae in ant-excluded colonies. A few P. lewisii adults were noted only in P. pungens-attended colonies, whereas some S. posticalis adults were observed in ant-excluded colonies. In most encounters, P. pungens workers tapped P. lewisii larvae with their antennae but showed no aggressive behavior; otherwise, P. pungens workers ignored the larvae. P. pungens exhibited the same be- havior when encountering S. posticalis larvae. The proportion of P. pungens-attended aphid colonies where the larvae of both coccinellids occurred did not significantly differ from the probability of both coccinellids occurring in the same colonies given their random distribution across the colonies. -

Irish Ants (Hymenoptera, Formicidae): Distribution, Conservation and Functional Relationships
Irish Ants (Hymenoptera, Formicidae): Distribution, Conservation and Functional Relationships Submitted by: Dipl. Biol. Robin Niechoj Supervisor: Prof. John Breen Submitted in accordance with the academic requirements for the Degree of Doctor of Philosophy to the Department of Life Sciences, Faculty of Science and Engineering, University of Limerick Limerick, April 2011 Declaration I hereby declare that I am the sole author of this thesis and that it has not been submitted for any other academic award. References and acknowledgements have been made, where necessary, to the work of others. Signature: Date: Robin Niechoj Department of Life Sciences Faculty of Science and Engineering University of Limerick ii Acknowledgements/Danksagung I wish to thank: Dr. John Breen for his supervision, encouragement and patience throughout the past 5 years. His infectious positive attitude towards both work and life was and always will be appreciated. Dr. Kenneth Byrne and Dr. Mogens Nielsen for accepting to examine this thesis, all the CréBeo team for advice, corrections of the report and Dr. Olaf Schmidt (also) for verification of the earthworm identification, Dr. Siobhán Jordan and her team for elemental analyses, Maria Long and Emma Glanville (NPWS) for advice, Catherine Elder for all her support, including fieldwork and proof reading, Dr. Patricia O’Flaherty and John O’Donovan for help with the proof reading, Robert Hutchinson for his help with the freeze-drying, and last but not least all the staff and postgraduate students of the Department of Life Sciences for their contribution to my work. Ich möchte mich bedanken bei: Katrin Wagner für ihre Hilfe im Labor, sowie ihre Worte der Motivation. -

Analysis of Recent Interception Records Reveals Frequent Transport of Arboreal Ants and Potential Predictors for Ant Invasion in Taiwan
insects Article Analysis of Recent Interception Records Reveals Frequent Transport of Arboreal Ants and Potential Predictors for Ant Invasion in Taiwan 1 2 3 4,5,6 7, Ching-Chen Lee , Yi-Ming Weng , Li-Chuan Lai , Andrew V. Suarez , Wen-Jer Wu y , 8, 9,10,11, , Chung-Chi Lin y and Chin-Cheng Scotty Yang * y 1 Center for Ecology and Environment, Department of Life Science, Tunghai University, Taichung 40704, Taiwan; [email protected] 2 Department of Entomology, University of Wisconsin-Madison, Madison, WI 53706, USA; [email protected] 3 Department of Ecological Humanities, Providence University, Taichung 43301, Taiwan; [email protected] 4 Department of Entomology, University of Illinois, Urbana-Champaign, Urbana, IL 61801, USA; [email protected] 5 Department of Evolution, Ecology, and Behavior, University of Illinois, Urbana-Champaign, Urbana, IL 61801, USA 6 Beckman Institute for Science and Technology, Urbana-Champaign, Urbana, IL 61801, USA 7 Department of Entomology, National Taiwan University, Taipei 10617, Taiwan; [email protected] 8 Department of Biology, National Changhua University of Education, Changhua 50007, Taiwan; [email protected] 9 Research Institute for Sustainable Humanosphere, Kyoto University, Kyoto 611-0011, Japan 10 Department of Entomology, Virginia Polytechnic Institute and State University, Blacksburg, VA 24061, USA 11 Department of Entomology, National Chung Hsing University, Taichung 402204, Taiwan * Correspondence: [email protected]; Tel.: +81-774-38-3874 These authors contributed equally to this work. y Received: 22 April 2020; Accepted: 4 June 2020; Published: 8 June 2020 Abstract: We uncovered taxonomic diversity, country of origin and commodity type of intercepted ants at Taiwanese borders based on an 8 year database of 439 interception records. -

Based Alpha-Taxonomy of Eusocial Organisms
Myrmecological News 19 1-15 Vienna, January 2014 Application of Exploratory Data Analyses opens a new perspective in morphology- based alpha-taxonomy of eusocial organisms Bernhard SEIFERT, Markus RITZ & Sándor CSŐSZ Abstract This article introduces a new application of the Exploratory Data Analysis (EDA) algorithms Ward's method, Unweighted Pair Group Method with Arithmetic Mean (UPGMA), K-Means clustering, and a combination of Non-Metric Multidimen- sional Scaling and K-Means clustering (NMDS-K-Means) for hypothesis formation in morphology-based alpha-taxonomy of ants. The script is written in R and freely available at: http://sourceforge.net/projects/agnesclustering/. The characte- ristic feature of the new approach is an unconventional application of linear discriminant analysis (LDA): No species hypothesis is imposed. Instead each nest sample, composed of individual ant workers, is treated as a separate class. This creates a multidimensional distance matrix between group centroids of nest samples as input data for the clustering methods. We mark the new method with the prefix "NC" (Nest Centroid). The performance of NC-Ward, NC-UPGMA, NC-K-Means clustering, and a combination of Non-Metric Multidimensional Scaling and K-Means clustering (NC- NMDS-K-Means) was comparatively tested in 48 examples with multiple morphological character sets of 74 cryptic species of 13 ant genera. Data sets were selected specifically on the criteria that the EDA methods are likely to lead to errors – i.e., for the condition that any character under consideration overlapped interspecifically in bivariate plots against body size. Morphospecies hypotheses were formed through interaction between EDA and a confirmative linear discri- minant analysis (LDA) in which samples with disagreements between the primary species hypotheses and EDA classifica- tion were set as wild-cards. -

Scientific Name Common Name Taxon Group Aceria Pseudoplatani
Scientific Name Common Name Taxon Group Aceria pseudoplatani Aceria pseudoplatani acarine (Acari) Alabidocarpus acarine (Acari) Phytoptus avellanae Phytoptus avellanae acarine (Acari) Tetranychidae Tetranychidae acarine (Acari) Tetranychus urticae Tetranychus urticae acarine (Acari) Trombidiidae Trombidiidae acarine (Acari) Bufo bufo Common Toad amphibian Lissotriton helveticus Palmate Newt amphibian Lissotriton vulgaris Smooth Newt amphibian Rana Frog amphibian Rana temporaria Common Frog amphibian Triturus Newt amphibian Triturus cristatus Great Crested Newt amphibian Triturus helveticus Palmate Newt amphibian Annelida Worms annelid Eisenia fetida Manure Worm annelid Erpobdella testacea Erpobdella testacea annelid Lumbricus terrestris Common Earthworm annelid Acanthis flammea/cabaret agg. Redpoll (Common\Lesser) agg. bird Accipiter nisus Sparrowhawk bird Acrocephalus schoenobaenus Sedge Warbler bird Aegithalos caudatus Long-tailed Tit bird Alauda arvensis Skylark bird Anas platyrhynchos Mallard bird Anthus pratensis Meadow Pipit bird Apodidae Swifts bird Apus apus Swift bird Scientific Name Common Name Taxon Group Ardea cinerea Grey Heron bird Aythya fuligula Tufted Duck bird Branta canadensis Canada Goose bird Buteo buteo Buzzard bird Caprimulgus europaeus Nightjar bird Carduelis carduelis Goldfinch bird Certhia familiaris Treecreeper bird Chloris chloris Greenfinch bird Chroicocephalus ridibundus Black-headed Gull bird Cinclus cinclus Dipper bird Coloeus monedula Jackdaw bird Columba Pigeon bird Columba livia Feral Pigeon/Rock Dove