Irish Ants (Hymenoptera, Formicidae): Distribution, Conservation and Functional Relationships
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Impact of Afforestation on Earthworm Populations in Iceland
ICEL. AGRIC. SCI. 26 (2013), 21-36 Impact of afforestation on earthworm populations in Iceland Bjarni D. SigurDSSon and Bjarni E. guDlEifSSon Agricultural University of Iceland, Hvanneyri, IS-311 Borgarnes, Iceland. e-mail: [email protected] (corresponding author), [email protected] ABSTRACT Earthworms were collected from different vegetation types in East and West Iceland. The vegetation types in East Iceland were Siberian larch (Larix sibirica) forests, native mountain birch (Betula pubescens) wood- lands and open heathlands. The study areas in West Iceland were Sitka spruce (Picea sitchensis), lodgepole pine (Pinus contorta) forests, mountain birch woodlands and open heathlands. Four earthworm species (Dendrobaena octaedra, Dendrodrilus rubidus, Aporrectodea caliginosa, Lumbricus rubellus) were identi- fied at both study areas and two additional ones in the West Iceland (Aporrectodea rosea and Octolasion cyaneum). No significant differences were detected in average earthworm species number and biomass between treeless heathlands and forests in East or West Iceland. There were, however, significant differences between the native deciduous forests and the coniferous plantations in West, but not East Iceland. Time since afforestation was found to have a significant effect on both earthworm diversity and density and should always be included in future studies. All earthworm parameters were positively related to soil N and amount of monocots, but negatively related to soil C/N ratio, tree LAI and tree height. Soil pH had no significant influence on any of the earthworm parameters. The most noteworthy finding was that earthworms were generally found in similar biomass and species richness in the exotic coniferous plantations in Iceland compared to the treeless heathlands, even if earthworm species composition showed strong changes. -

Annelida, Lumbricidae) - Description Based on Morphological and Molecular Data
A new earthworm species within a controversial genus: Eiseniona gerardoi sp. n. (Annelida, Lumbricidae) - description based on morphological and molecular data The Harvard community has made this article openly available. Please share how this access benefits you. Your story matters Citation Díaz Cosín, Darío J., Marta Novo, Rosa Fernández, Daniel Fernández Marchán, and Mónica Gutiérrez. 2014. “A new earthworm species within a controversial genus: Eiseniona gerardoi sp. n. (Annelida, Lumbricidae) - description based on morphological and molecular data.” ZooKeys (399): 71-87. doi:10.3897/zookeys.399.7273. http:// dx.doi.org/10.3897/zookeys.399.7273. Published Version doi:10.3897/zookeys.399.7273 Citable link http://nrs.harvard.edu/urn-3:HUL.InstRepos:12406906 Terms of Use This article was downloaded from Harvard University’s DASH repository, and is made available under the terms and conditions applicable to Other Posted Material, as set forth at http:// nrs.harvard.edu/urn-3:HUL.InstRepos:dash.current.terms-of- use#LAA A peer-reviewed open-access journal ZooKeys 399: A71–87 new (2014) earthworm species within a controversial genus: Eiseniona gerardoi sp. n... 71 doi: 10.3897/zookeys.399.7273 RESEARCH ARTICLE www.zookeys.org Launched to accelerate biodiversity research A new earthworm species within a controversial genus: Eiseniona gerardoi sp. n. (Annelida, Lumbricidae) - description based on morphological and molecular data Darío J. Díaz Cosín1,†, Marta Novo1,2,‡, Rosa Fernández1,3,§, Daniel Fernández Marchán1,|, Mónica Gutiérrez1,¶ 1 -

Killarney, Barraduff & Gneeveguilla Wwtp Discharges Natura Impact Statement
KERRY COUNTY COUNCIL COMHAIRLE CONTAE CHIARRAÍ KILLARNEY, BARRADUFF & GNEEVEGUILLA WWTP DISCHARGES NATURA IMPACT STATEMENT For inspection purposes only. Consent of copyright owner required for any other use. JANUARY 2010 CONSULTING ENGINEERS Sherwood House, Sherwood Avenue, Taylor’s Hill, Galway Suite D4, The Cubes Offices, Beacon South Quarter, Sandyford Dublin 18 EPA Export 04-11-2011:03:50:18 Quality Control CLIENT Kerry County Council PROJECT NO 2178 PROJECT TITLE KILLARNEY, BARRADUFF & GNEEVEGUILLA WWTP DISCHARGES REPORT TITLE NATURA IMPACT STATEMENT Rev. Status Author(s) Reviewed By Approved By Issue Date 1 ISSUE GH MJ MJ 04.03.2011 For inspection purposes only. Consent of copyright owner required for any other use. Natura Impact Statement Page 2 EPA Export 04-11-2011:03:50:18 TABLE OF CONTENTS 1 INTRODUCTION ............................................................................................ 4 1.1 BACKGROUND.....................................................................................................................4 1.2 APPROPRIATE ASSESSMENT – LEGISLATIVE CONTEXT...............................................5 1.3 NATURA 2000 SITES ...........................................................................................................7 1.4 SCHEME LOCATIONS..........................................................................................................8 2 METHODOLOGY.......................................................................................... 11 2.1 DESK STUDY...................................................................................................................... -

Lasius Fuliginosus (Hymenoptera: Formicidae) Shapes Local Ant Assemblages
NORTH-WESTERN JOURNAL OF ZOOLOGY 10 (2): 404-412 ©NwjZ, Oradea, Romania, 2014 Article No.: 141104 http://biozoojournals.ro/nwjz/index.html Lasius fuliginosus (Hymenoptera: Formicidae) shapes local ant assemblages Piotr ŚLIPIŃSKI1,*, Bálint MARKÓ2, Kamil RZESZOWSKI1, Hanna BABIK1 and Wojciech CZECHOWSKI1 1. Museum and Institute of Zoology, Polish Academy of Sciences, Wilcza 64, 00-679 Warsaw, Poland, E-mails: [email protected], [email protected], [email protected], [email protected]. 2. Hungarian Department of Biology and Ecology, Babeş-Bolyai University, Clinicilor str. 5-7, 400006 Cluj-Napoca, Romania, E-mail: [email protected]. * Corresponding author, P. Ślipiński, E-mail: [email protected] Received: 20. December 2013 / Accepted: 22. March 2014 / Available online: 17. October 2014 / Printed: December 2014 Abstract. Interspecific competition is a major structuring force in ant assemblages. The assemblages are organized hierarchically, with territorial species as top competitors. In boreal areas and in the temperate deciduous forest biome common territorials are species of the subgenus Formica s. str. They are well known for their negative impact on lower-ranked ant species. Less is known, though the structuring role of Lasius fuliginosus, another territorial ant species. Some earlier studies have shown or suggested that it may restrictively affect subordinate species (including direct predation toward them) even stronger than wood ants do. In the present study we compared species compositions and nest densities of subordinate ant species within and outside territories of L. fuliginosus. The results obtained confirmed that this species visibly impoverishes both qualitatively (reduced species richness, altered dominance structures) and quantitatively (decreased nest densities) ant assemblages within its territories. -
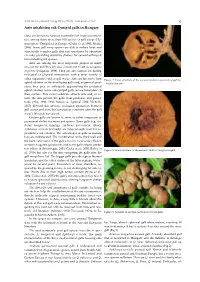
Ants Inhabiting Oak Cynipid Galls in Hungary
North-Western Journal of Zoology 2020, vol.16 (1) - Correspondence: Notes 95 Ants inhabiting oak Cynipid galls in Hungary Oaks are known to harbour extremely rich insect communi- ties, among them more than 100 species of gall wasps (Hy- menoptera: Cynipidae) in Europe (Csóka et al. 2005, Melika 2006). Some gall wasp species are able to induce large and structurally complex galls that can sometimes be abundant on oaks, providing attractive shelters for several arthropod taxa including ant species. Ants are among the most important players in many ecosystems and they are also considered to act as ecosystem engineers (Folgarait, 1998). They are also famous for having ecological or physical interactions with a great variety of other organisms, such as gall wasps. Ants are known to tend Figure 1. Inner structure of the asexual Andricus quercustozae gall in- aphid colonies on the developing galls and, as general pred- habited by ants. ators, they prey on arthropods approaching the protected aphid colonies. Some oak cynipid galls secrete honeydew on their surface. This sweet substrate attracts ants and, in re- turn, the ants protect the galls from predators and parasi- toids (Abe, 1988, 1992; Inouye & Agrawal 2004; Nicholls, 2017). Beyond this obvious ecological interaction between gall wasps and ants, this association continues after the gall wasp’s life cycle has ceased. Certain galls are known to serve as either temporary or permanent shelter for many ant species. Some galls (e.g. An- dricus hungaricus (Hartig), Andricus quercustozae (Bosc), Aphelonyx cerricola (Giraud)) are large enough even for re- productive ant colonies. The advantages of galls as nesting logs are multifaceted. -

409-456 Rejstriky Olomoucko1
Bibliografie 1. ALBRECHT, P. (1996–2000): Inventarizaãní prÛzkumy 19. BEZDùâKA, P. (1983): Lesní mravenci na lokalitû Alf- âubernice, Lipovské upolínové louky, Na HÛrkách, rédka. – Na‰í pfiírodou, 2: 18–19, Praha. Pod Pansk˘m lesem, Vitãick˘ les, Za Hrnãífikou. – 20. BEZDùâKA, P. (1998): Mravenci Pfiírodního parku Vel- Depon. in RÎP OkÚ Prostûjov, 25 s., 38 s., 17 s., 15 k˘ Kosífi. – Pfiírodovûdné studie Muzea Prostûjovska, s., 32 s., 25 s., Prostûjov. (nepubl.) 1: 125–132, Prostûjov. 2. ALBRECHT, P. (1998): Krajiny Prostûjovska. – Pfiírodo- 21. BEZDùâKA, P. (1999): V˘voj komplexu hnízd Formica vûdné studie Muzea Prostûjovska, 1: 47–66, Prostûjov. lugubris Zett. v Jeseníkách. – Formica, zpravodaj pro 3. ALBRECHT, P. (1999): Fytocenologické snímky moni- aplikovan˘ v˘zkum a ochranu lesních mravencÛ, 2: torovacích ploch LIPO 02.01 a 02.02. Správa CHKO 65–70, Liberec. Litovelské Pomoraví, 3 s., Olomouc. (nepubl.) 22. BUâEK, A. (1979): Pfiíspûvek k inventarizaci SPR Bu- 4. ALBRECHT, P. (2000): Pfiíspûvek ke kvûtenû nejvy‰‰ích koveãek. MS, práce SOâ pfii SLT· Hranice, 18 s., ãástí Drahanské vrchoviny se zamûfiením na mokfiady. Hranice. – Pfiírodovûdné studie Muzea Prostûjovska, 3: 55–86, 23. BURE·, L., BURE·OVÁ, Z. (1988): âerven˘ seznam cév- Prostûjov. nat˘ch rostlin CHKO Jeseníky. – Zpravodaj âSOP, 3: 5. ANDùRA, M. (1999): âeské názvy ÏivoãichÛ II. Savci 1–28, Bruntál. (Mammalia). Národní muzeum, 148 s., Praha. 24. BURE·, L., BURE·OVÁ, Z. (1989): Velká kotlina, státní 6. ANDùRA, M., HANZAL, V. (1996): Atlas roz‰ífiení sav- pfiírodní rezervace. – PrÛvodce nauãnou stezkou, cÛ v âeské republice. PfiedbûÏná verze. II: ·elmy (Car- âSOP ZO, 44 s., Ostrava. nivora). -

Download PDF File (107KB)
Myrmecological News 15 Digital supplementary material Digital supplementary material to SEPPÄ, P., HELANTERÄ, H., TRONTTI, K., PUNTTILA, P., CHERNENKO, A., MARTIN, S.J. & SUNDSTRÖM, L. 2011: The many ways to delimit species: hairs, genes and surface chemistry. – Myrmecological News 15: 31-41. Appendix 1: Number of hairs on different body parts of Formica fusca and F. lemani according to different authors. Promesonotum & pronotum YARROW COLLINGWOOD DLUSSKY & KUTTER COLLINGWOOD DOUWES CZECHOWSKI & SEIFERT SEIFERT (1954) (1958) PISARSKI (1971) (1977) (1979) (1995) al. (2002) (1996) (2007) F. fusca < 3 at most 2 - 3 ≤ 2 usually 0, usually = 0, ≤ 2 usually 0, rarely average average sometimes occasion. 1 - 2 1 - 5 < 1 0 - 0.8 1 - 4 F. lemani numerous numerous some ind. >10, up to 20 "with erect ≥ 3 > 6 average average in SE-Europ. popu- hairs" > 1 1.2 - 13.5 lations, most ind. 0 Femora F. fusca mid = 0 mid = 0 mid = 0 mid ≤ 1 fore = 2 - 3 hind = 0 hind = 0 mid = rarely 1 - 2 F. lemani mid = "long all = "hairy" mid = a few mid ≥ 2 fore = 3 - 12 hairs" hind = a few mid = 3 - 17 References COLLINGWOOD, C.A. 1958: A key to the species of ants (Hymenoptera, Formicidae) found in Britain. – Transactions of the Society for British Entomology 13: 69-96. COLLINGWOOD, C.A. 1979: The Formicidae (Hymenoptera) of Fennoscandia and Denmark. – Fauna Entomologica Scandinavica 8: 1-174. CZECHOWSKI, W., RADCHENKO, A. & CZECHOWSKA, W. 2002: The ants (Hymenoptera, Formicidae) of Poland. – Museum and Insti- tute of Zoology PAS, Warszawa, 200 pp. DLUSSKY, G.M. & PISARSKI, B. 1971: Rewizja polskich gatunków mrówek (Hymenoptera: Formicidae) z rodzaju Formica L. -

Download PDF ( Final Version , 500Kb )
infecties met platwrat AEGERITELLA TUBERCULATA op schubmieren (formicidae: formicinae) Peter Boer & Jinze Noordijk Mieren kunnen geïnfecteerd zijn met specifieke schimmels, zoals die van het genus Aegeritella. Platwrat Aegiterella tuberculata was uit de literatuur bekend van 12 mierensoorten uit Europa en één uit Noord-Amerika. In Nederland troffen wij tot nu toe zes soorten aan met deze infectie, waarvan Lasius alienus en Formica exsecta hier als nieuwe gastheer worden gemeld. Bovendien kregen we Grieks materiaal onder ogen, waaruit bleek dat platwrat ook aanwezig is op de invasieve exoot Lasius neglectus. Het aantal bekende gastheren komt daarmee op 16. Ectoparasitaire schimmels op mieren worden nauwelijks onderzocht en er is ongetwijfeld nog veel te ontdekken aan de waardsoorten, de verspreiding en de mogelijke nadelige effecten op mieren. schimmels op mieren schoonvegen met antibiotische uitscheidings- Mieren hebben een goed werkend afweersysteem producten uit de metapleurale klieren (Beattie voor allerlei ziekmakende bacteriën en schimmels. et al. 1986). Ongerechtigheden zoals bijvoorbeeld Dat komt doordat ze zichzelf en elkaar likken en schimmelsporen worden in een holte voor in de Figuur 1. Werkster van Lasius umbratus met infectie van Aegeritella tuberculata. Posterholt (Holst, Limburg), .vi.2013, leg. Jinze Noordijk. Foto Theodoor Heijerman. Figure 1. Worker of Lasius umbratus with infection of Aegeritella tuberculata. Posterholt (Holst, province of Limburg, the Netherlands), .vi.2013, leg. Jinze Noordijk. Photo Theodoor Heijerman. boer & noordijk ‒ platwrat op schubmieren Figuur 2. Werkster van Formica polyctena met infectie van Aegeritella superficialis. Oranje Nassau’s Oord (Wageningen, Gelderland), 16.iii.2014, leg. Theodoor Heijerman. Foto Theodoor Heijerman. Figure 2. Worker of Formica polyctena with infection of Aegeritella superficialis. -

Akes an Ant an Ant? Are Insects, and Insects Are Arth Ropods: Invertebrates (Animals With
~ . r. workers will begin to produce eggs if the queen dies. Because ~ eggs are unfertilized, they usually develop into males (see the discus : ~ iaplodiploidy and the evolution of eusociality later in this chapter). =- cases, however, workers can produce new queens either from un ze eggs (parthenogenetically) or after mating with a male ant. -;c. ant colony will continue to grow in size and add workers, but at -: :;oint it becomes mature and will begin sexual reproduction by pro· . ~ -irgin queens and males. Many specie s produce males and repro 0 _ " females just before the nuptial flight . Others produce males and ---: : ._ tive fem ales that stay in the nest for a long time before the nuptial :- ~. Our largest carpenter ant, Camponotus herculeanus, produces males _ . -:= 'n queens in late summer. They are groomed and fed by workers :;' 0 it the fall and winter before they emerge from the colonies for their ;;. ights in the spring. Fin ally, some species, including Monomoriurn : .:5 and Myrmica rubra, have large colonies with multiple que ens that .~ ..ew colonies asexually by fragmenting the original colony. However, _ --' e polygynous (literally, many queens) and polydomous (literally, uses, referring to their many nests) ants eventually go through a -">O=- r' sexual reproduction in which males and new queens are produced. ~ :- . ant colony thus functions as a highly social, organ ized "super _ _ " 1." The queens and mo st workers are safely hidden below ground : : ~ - ed within the interstices of rotting wood. But for the ant workers ~ '_i S ' go out and forage for food for the colony,'life above ground is - =- . -

Annelida, Lumbricidae) - Description Based on Morphological and Molecular Data
A peer-reviewed open-access journal ZooKeys 399: A71–87 new (2014) earthworm species within a controversial genus: Eiseniona gerardoi sp. n... 71 doi: 10.3897/zookeys.399.7273 RESEARCH ARTICLE www.zookeys.org Launched to accelerate biodiversity research A new earthworm species within a controversial genus: Eiseniona gerardoi sp. n. (Annelida, Lumbricidae) - description based on morphological and molecular data Darío J. Díaz Cosín1,†, Marta Novo1,2,‡, Rosa Fernández1,3,§, Daniel Fernández Marchán1,|, Mónica Gutiérrez1,¶ 1 Departamento de Zoología y Antropología Física, Facultad de Biología, Universidad Complutense de Madrid, C/ José Antonio Nováis 2, 28040, Madrid, Spain 2 Cardiff School of Biosciences, Cardiff University, BIOSI 1, Museum Avenue, Cardiff CF10, 3TL, UK3 Museum of Comparative Zoology, Department of Organismic and Evolutionary Biology, Harvard University, 26 Oxford Street, Cambridge, MA 02138, USA † http://zoobank.org/38538B17-F127-4438-9DE2-F9D6C597D044 ‡ http://zoobank.org/79DA5419-91D5-4EAB-BC72-1E46F10C716A § http://zoobank.org/99618966-BB50-4A01-8FA0-7B1CC31686B6 | http://zoobank.org/CAB83B57-ABD1-40D9-B16A-654281D71D58 ¶ http://zoobank.org/E1A7E77A-9CD5-4D67-88A3-C7F65AD6A5BE Corresponding author: Darío J. Díaz Cosín ([email protected]) Academic editor: R. Blakemore | Received 17 February 2014 | Accepted 25 March 2014 | Published 9 April 2014 http://zoobank.org/F5AC3116-E79E-4442-9B26-2765A5243D5E Citation: Cosín DJD, Novo M, Fernández R, Marchán DF, Gutiérrez M (2014) A new earthworm species within a controversial genus: Eiseniona gerardoi sp. n. (Annelida, Lumbricidae) - description based on morphological and molecular data. ZooKeys 399: 71–87. doi: 10.3897/zookeys.399.7273 Abstract The morphological and anatomical simplicity of soil dwelling animals, such as earthworms, has limited the establishment of a robust taxonomy making it sometimes subjective to authors’ criteria. -

Bee Viruses: Routes of Infection in Hymenoptera
fmicb-11-00943 May 27, 2020 Time: 14:39 # 1 REVIEW published: 28 May 2020 doi: 10.3389/fmicb.2020.00943 Bee Viruses: Routes of Infection in Hymenoptera Orlando Yañez1,2*, Niels Piot3, Anne Dalmon4, Joachim R. de Miranda5, Panuwan Chantawannakul6,7, Delphine Panziera8,9, Esmaeil Amiri10,11, Guy Smagghe3, Declan Schroeder12,13 and Nor Chejanovsky14* 1 Institute of Bee Health, Vetsuisse Faculty, University of Bern, Bern, Switzerland, 2 Agroscope, Swiss Bee Research Centre, Bern, Switzerland, 3 Laboratory of Agrozoology, Department of Plants and Crops, Faculty of Bioscience Engineering, Ghent University, Ghent, Belgium, 4 INRAE, Unité de Recherche Abeilles et Environnement, Avignon, France, 5 Department of Ecology, Swedish University of Agricultural Sciences, Uppsala, Sweden, 6 Environmental Science Research Center, Faculty of Science, Chiang Mai University, Chiang Mai, Thailand, 7 Department of Biology, Faculty of Science, Chiang Mai University, Chiang Mai, Thailand, 8 General Zoology, Institute for Biology, Martin-Luther-University of Halle-Wittenberg, Halle (Saale), Germany, 9 Halle-Jena-Leipzig, German Centre for Integrative Biodiversity Research (iDiv), Leipzig, Germany, 10 Department of Biology, University of North Carolina at Greensboro, Greensboro, NC, United States, 11 Department Edited by: of Entomology and Plant Pathology, North Carolina State University, Raleigh, NC, United States, 12 Department of Veterinary Akio Adachi, Population Medicine, College of Veterinary Medicine, University of Minnesota, Saint Paul, MN, United States, -

Sistemática Y Ecología De Las Hormigas Predadoras (Formicidae: Ponerinae) De La Argentina
UNIVERSIDAD DE BUENOS AIRES Facultad de Ciencias Exactas y Naturales Sistemática y ecología de las hormigas predadoras (Formicidae: Ponerinae) de la Argentina Tesis presentada para optar al título de Doctor de la Universidad de Buenos Aires en el área CIENCIAS BIOLÓGICAS PRISCILA ELENA HANISCH Directores de tesis: Dr. Andrew Suarez y Dr. Pablo L. Tubaro Consejero de estudios: Dr. Daniel Roccatagliata Lugar de trabajo: División de Ornitología, Museo Argentino de Ciencias Naturales “Bernardino Rivadavia” Buenos Aires, Marzo 2018 Fecha de defensa: 27 de Marzo de 2018 Sistemática y ecología de las hormigas predadoras (Formicidae: Ponerinae) de la Argentina Resumen Las hormigas son uno de los grupos de insectos más abundantes en los ecosistemas terrestres, siendo sus actividades, muy importantes para el ecosistema. En esta tesis se estudiaron de forma integral la sistemática y ecología de una subfamilia de hormigas, las ponerinas. Esta subfamilia predomina en regiones tropicales y neotropicales, estando presente en Argentina desde el norte hasta la provincia de Buenos Aires. Se utilizó un enfoque integrador, combinando análisis genéticos con morfológicos para estudiar su diversidad, en combinación con estudios ecológicos y comportamentales para estudiar la dominancia, estructura de la comunidad y posición trófica de las Ponerinas. Los resultados sugieren que la diversidad es más alta de lo que se creía, tanto por que se encontraron nuevos registros durante la colecta de nuevo material, como porque nuestros análisis sugieren la presencia de especies crípticas. Adicionalmente, demostramos que en el PN Iguazú, dos ponerinas: Dinoponera australis y Pachycondyla striata son componentes dominantes en la comunidad de hormigas. Análisis de isótopos estables revelaron que la mayoría de las Ponerinas ocupan niveles tróficos altos, con excepción de algunas especies arborícolas del género Neoponera que dependerían de néctar u otros recursos vegetales.