The Effect of Heat Exchange Fluid Composition on the Performance of a Liquid Nitrogen Engine System
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Liquid Argon
Safetygram 8 Liquid argon Liquid argon is tasteless, colorless, odorless, noncorrosive, nonflammable, and extremely cold. Belonging to the family of rare gases, argon is the most plentiful, making up approximately 1% of the earth’s atmosphere. It is monatomic and extremely inert, forming no known chemical compounds. Since argon is inert, special materials of construction are not required. However, materials of construction must be selected to withstand the low temperature of liquid argon. Vessels and piping should be designed to American Society of Mechanical Engineers (ASME) specifications or the Department of Transportation (DOT) codes for the pressures and temperatures involved. Although used more commonly in the gaseous state, argon is commonly stored and transported as a liquid, affording a more cost-effective way of providing product supply. Liquid argon is a cryogenic liquid. Cryogenic liquids are liquefied gases that have a normal boiling point below –130°F (–90°C). Liquid argon has a boiling point of –303°F (–186°C). The temperature difference between the product and the surrounding environment, even in winter, is substantial. Keeping this surrounding heat from the product requires special equipment to store and handle cryogenic liquids. A typical system consists of the following components: a cryogenic storage tank, one or more vaporizers, a pressure control system, and all of the piping required for fill, vaporization. The cryogenic tank is constructed, in principle, like a vacuum bottle. It is designed to keep heat away from the liquid that is contained in the inner vessel. Vaporizers convert the liquid argon to its gaseous state. A pressure control manifold controls the pressure at which the gas is fed to the process. -

Cryogenic Liquid Nitrogen Vehicles (ZEV's)
International Journal of Scientific and Research Publications, Volume 6, Issue 9, September 2016 562 ISSN 2250-3153 Cryogenic Liquid Nitrogen Vehicles (ZEV’S) K J Yogesh Department Of Mechanical Engineering, Jain Engineering College, Belagavi Abstract- As a result of widely increasing air pollution available zero emission vehicle (ZEV) meeting it's standards are throughout the world & vehicle emissions having a major the electrically recharged ones, however these vehicles are also contribution towards the same, it makes its very essential to not a great success in the society due to its own limitations like engineer or design an alternative to the present traditional initial cost, slow recharge, speeds etc. Lead acid & Ni-Cd gasoline vehicles. Liquid nitrogen fueled vehicles can act as an batteries are the past of major technologies in the electric excellent alternative for the same. Liquefied N2 at cryogenic vehicles. They exhibit specific energy in the range of 30-40 W- temperatures can replace conventional fuels in cryogenic heat hr/kg. Lead- acid batteries take hours to recharge & the major engines used as a propellant. The ambient temperature of the drawback of the batteries in all the cases is their replacement surrounding vaporizes the liquid form of N2 under pressure & periodically. This directly/indirectly increases the operating cost leads to the formation of compressed N2 gas. This gas actuates a when studied carefully & thereby not 100% acceptable. pneumatic motor. A combination of multiple reheat open Recent studies make it clear that the vehicles using liquid Rankine cycle & closed Brayton cycle are involved in the nitrogen as their means provide an excellent alternative before process to make use of liquid N2 as a non-polluting fuel. -

Capacity Enhancement of Indigenous Expansion Engine Based Helium Liquefier
IOP Conference Series: Materials Science and Engineering PAPER • OPEN ACCESS Capacity enhancement of indigenous expansion engine based helium liquefier To cite this article: R S Doohan et al 2017 IOP Conf. Ser.: Mater. Sci. Eng. 171 012011 View the article online for updates and enhancements. This content was downloaded from IP address 170.106.202.8 on 27/09/2021 at 00:16 ICECICMC IOP Publishing IOP Conf. Series: Materials Science and Engineering 171 (2017) 012011 doi:10.1088/1757-899X/171/1/012011 International Conference on Recent Trends in Physics 2016 (ICRTP2016) IOP Publishing Journal of Physics: Conference Series 755 (2016) 011001 doi:10.1088/1742-6596/755/1/011001 Capacity enhancement of indigenous expansion engine based helium liquefier R S Doohan1, P K Kush1, G Maheshwari2 1Raja Ramanna Centre for Advanced Technology, Indore, Madhya Pradesh India. 2Institute of Engineering and Technology, DAVV, Indore, Madhya Pradesh, India. E-mail: [email protected] Abstract. Development of technology and understanding for large capacity helium refrigeration and liquefaction at helium temperature is indispensable for coming-up projects. A new version of helium liquefier designed and built to provide approximately 35 liters of liquid helium per hour. The refrigeration capacity of this reciprocating type expansion engine machine has been increased from its predecessor version with continuous improvement and deficiency debugging. The helium liquefier has been built using components by local industries including cryogenic Aluminum plate fin heat exchangers. Two compressors with nearly identical capacity have been deployed for the operation of system. Together they consume about 110 kW of electric power. The system employs liquid Nitrogen precooling to enhance liquid Helium yield. -
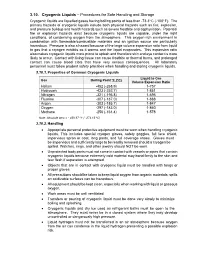
3.10. Cryogenic Liquids – Procedures for Safe Handling and Storage Cryogenic Liquids Are Liquefied Gases Having Boiling Points of Less Than -73.3O C (-100O F)
3.10. Cryogenic Liquids – Procedures for Safe Handling and Storage Cryogenic liquids are liquefied gases having boiling points of less than -73.3o C (-100o F). The primary hazards of cryogenic liquids include both physical hazards such as fire, explosion, and pressure buildup and health hazards such as severe frostbite and asphyxiation. Potential fire or explosion hazards exist because cryogenic liquids are capable, under the right conditions, of condensing oxygen from the atmosphere. This oxygen-rich environment in combination with flammable/combustible materials and an ignition source are particularly hazardous. Pressure is also a hazard because of the large volume expansion ratio from liquid to gas that a cryogen exhibits as it warms and the liquid evaporates. This expansion ratio also makes cryogenic liquids more prone to splash and therefore skin and eye contact is more likely to occur. Contact with living tissue can cause frostbite or thermal burns, and prolonged contact can cause blood clots that have very serious consequences. All laboratory personnel must follow prudent safety practices when handling and storing cryogenic liquids. 3.10.1. Properties of Common Cryogenic Liquids Liquid to Gas Gas Boiling Point oF (oC) Volume Expansion Ratio Helium -452 (-268.9) 1-757 Hydrogen -423 (-252.7) 1-851 Nitrogen -321 (-195.8) 1-696 Fluorine -307 (-187.0) 1-888 Argon -303 (-185.7) 1-847 Oxygen -297 (-183.0) 1-860 Methane -256 (-161.4) 1-578 Note: Absolute zero = - 459.67 oF (- 273.15 oC) 3.10.2. Handling Appropriate personal protective equipment must be worn when handling cryogenic liquids. -

Cryogenic Liquid Safety
Cryogenic Liquid Safety Hazards and Safe Handling of Cryogenic Liquids Cryogenic liquids have boiling points less than -73ºC (-100ºF). Liquid nitrogen, liquid oxygen and carbon dioxide are the most common cryogenic materials used in the laboratory. Hazards may include fire, explosion, embrittlement, pressure buildup, frostbite and asphyxiation. Many of the safety precautions observed for compressed gases also apply to cryogenic liquids. There are also additional hazards due to the unique properties of cryogenic liquids: • Extremely Low Temperatures: The cold boil-off vapor of cryogenic liquids rapidly freezes human tissue. Most metals become stronger upon exposure to cold temperatures, but materials, such as; carbon steel, plastics and rubber become brittle or fracture under stress at these temperatures. Proper material selection is important. Cold burns and frostbite caused by cryogenic liquids can result in extensive tissue damage. • Vaporization: All cryogenic liquids produce large volumes of gas when they vaporize. Liquid nitrogen will expand 696 times as it vaporizes. The expansion ratio of argon is 847:1, hydrogen is 851:1 and oxygen is 862:1. If these liquids vaporize in a sealed container, they can produce enormous pressures that could rupture the vessel. For this reason, pressurized cryogenic containers are usually protected with multiple pressure relief devices. • Vaporization of cryogenic liquids (except oxygen) in an enclosed area can cause asphyxiation! • Vaporization of liquid O2 can produce an oxygen-rich atmosphere, supporting and accelerating combustion of other materials. Vaporization of liquid hydrogen can form an extremely flammable mixture with air. Most cryogenic liquids are odorless, colorless, and tasteless when vaporized. When cryogenic liquids are exposed to the atmosphere, cold boil-off gases condense the moisture in the air, creating a highly visible fog. -

Battery Technologies for Small Scale Embeded Generation
Battery Technologies for Small Scale Embedded Generation. by Norman Jackson, South African Energy Storage Association (SAESA) Content Provider – Wikipedia et al Small Scale Embedded Generation - SSEG • SSEG is very much a local South African term for Distributed Generation under 10 Mega Watt. Internationally they refer to: Distributed generation, also distributed energy, on-site generation (OSG) or district/decentralized energy It is electrical generation and storage performed by a variety of small, grid- connected devices referred to as distributed energy resources (DER) Types of Energy storage: • Fossil fuel storage • Thermal • Electrochemical • Mechanical • Brick storage heater • Compressed air energy storage • Cryogenic energy storage (Battery Energy • Fireless locomotive • Liquid nitrogen engine Storage System, • Flywheel energy storage • Eutectic system BESS) • Gravitational potential energy • Ice storage air conditioning • Hydraulic accumulator • Molten salt storage • Flow battery • Pumped-storage • Phase-change material • Rechargeable hydroelectricity • Seasonal thermal energy battery • Electrical, electromagnetic storage • Capacitor • Solar pond • UltraBattery • Supercapacitor • Steam accumulator • Superconducting magnetic • Thermal energy energy storage (SMES, also storage (general) superconducting storage coil) • Chemical • Biological • Biofuels • Glycogen • Hydrated salts • Starch • Hydrogen storage • Hydrogen peroxide • Power to gas • Vanadium pentoxide History of the battery This was a stack of copper and zinc Italian plates, -

Liquid Helium
Safetygram 22 Liquid helium Liquid helium is inert, colorless, odorless, noncorrosive, extremely cold, and nonflammable. Helium will not react with other elements or compounds under ordinary conditions. Since helium is noncorrosive, special materials of construction are not required. However, materials must be suitable for use at the extremely low temperatures of liquid helium. Vessels and piping must be selected and designed to withstand the pressure and temperatures involved and comply with applicable codes for transport and use. Manufacture Most of commercial helium is recovered from natural gas through a cryogenic separation process. Normally, helium is present in less than 1% by volume in natural gas. Helium is recovered, refined, and liquefied. Liquid helium is typically shipped from production sources to storage and transfill facilities. Tankers, ranging in size from 5,000 to 11,000 gallons, contain an annular space insulated with vacuum, nitrogen shielding, and multilayer insulation. This de- sign reduces heat leak and vaporization of liquid helium during transportation. Uses The extremely low temperature of liquid helium is utilized to maintain the superconducting properties of magnets in applications such as MRI, NMR spectroscopy, and particle physics research. The main application for gas- eous helium is for inert shielding gas in metal arc and laser welding. Helium provides a protective atmosphere in the production of reactive metals, such as titanium and zirconium. Gaseous helium is used as a coolant during the draw- ing of optical fibers, as a carrier gas for chromatography, and as a leak detection gas in a variety of industries. Being both lighter than air and nonflammable, helium is used to inflate balloons and airships. -

Liquid Nitrogen Safety & Handling
Liquid Nitrogen Safety & Handling 1.0 Liquid Nitrogen & Cryogenic Liquids a. Liquid nitrogen, LN2, is colorless, odorless and tasteless. It is non-flammable, physiologically inert and non-toxic. It is an extremely cold liquid with a boiling point of - 196ºC = -320ºF. b. Cryogenic liquids are liquefied gases that are kept in their liquid state at extremely low temperatures. Cryogenic liquids have boiling points below -150°C (-238°F) (Carbon dioxide and nitrous oxide, which have slightly higher boiling points are sometimes included in this category). All cryogenic liquids are gases at normal temperatures and pressures. All Cryogenic liquids produce large amounts of gas when they vaporize. c. There a many applications of LN2 in a biomedical research facility, one of the most common being cryopreservation, the storage of cells at temperatures below -130 ºC, which is essential in order to preserve biological material unaltered. 2.0 Safety Hazards Associated with Handling LN2 a. The extremely low temperature of the liquid can cause severe frostbite or eye damage upon contact. Symptoms of frostbite include change in skin color to white or grayish yellow and the pain after contact with LN2 may quickly subside. Items in contact with liquid nitrogen become extremely cold. Touching these items may result in torn flesh. Many substances become brittle upon contact with liquid nitrogen and may shatter when cold (such as common glass and large solid plastics), sending pieces of the material flying. b. Upon vaporization it expands by a factor of 700; one liter of liquid nitrogen becomes 24.6 cubic feet of nitrogen gas. -

Liquid Oxygen
Safetygram 6 Liquid oxygen Oxygen is the second largest component of the atmosphere, comprising 20.8% by volume. Liquid oxygen is pale blue and extremely cold. Although nonflammable, oxygen is a strong oxidizer. Oxygen is necessary to support life. Oxygen will react with nearly all organic materials and metals, usually forming an oxide. Materials that burn in air will burn more vigorously in oxygen. Equipment used in oxygen service must meet stringent cleaning requirements, and systems must be constructed of materials that have high ignition temperatures and that are nonreactive with oxygen under the service conditions. Vessels should be manufactured to American Society of Mechanical Engineers (ASME) codes and designed to withstand the process temperatures and pressures. Liquid oxygen is a cryogenic liquid. Cryogenic liquids are liquefied gases that have a normal boiling point below –130°F (–90°C). Liquid oxygen has a boiling point of –297°F (–183°C). Because the temperature difference between the product and the surrounding environment is substantial—even in the winter—keeping liquid oxygen insulated from the surrounding heat is essential. The product also requires special equipment for handling and storage. Oxygen is often stored as a liquid, although it is used primarily as a gas. Liquid storage is less bulky and less costly than the equivalent capacity of high-pressure gaseous storage. A typical storage system consists of a cryogenic storage tank, one or more vaporizers and a pressure control system. The cryogenic tank is constructed, in principle, like a vacuum bottle. There is an inner vessel surrounded by an outer vessel. Between the vessels is an annular space that contains an insulating medium from which all the air has been removed. -

Birmingham Centre for Energy Storage
BIRMINGHAM CENTRE FOR ENERGY STORAGE @BHAMENERGY WWW.BIRMINGHAM.AC.UK/BCES 2 Birmingham Centre for Energy Storage Birmingham Centre for Energy Storage 3 BIRMINGHAM CENTRE ABOUT THE BIRMINGHAM FOR ENERGY ENERGY INSTITUTE STORAGE The Birmingham Energy Institute is a focal point for the University INTERNATIONAL THERMAL and its national and international partners to create change in the way we deliver, consume and think about energy. The Institute ENERGY MANUFACTURING harnesses expertise from the fundamental sciences and engineering Established in 2013, the Birmingham Centre for through to business and economics to deliver co-ordinated research, RESEARCH ACCELERATOR (ITEMA) ‘NEW STORAGE TECHNOLOGIES Energy Storage brings together research expertise education and the development of global partnerships. COULD TRANSFORM HOW ENERGY from across the University to drive innovation from the In partnership with Loughborough University, we are working with IS SUPPLIED AND USED, HELPING More than 140 academics across the University are engaged in the Manufacturing Technology Centre (MTC) to develop ITEMA; laboratory to market. The Centre received two strands TO MAKE FUTURE ENERGY SYSTEMS energy and energy related research and development, with over co-funded by Government, industry and universities. Leveraging Industry £75 million external research investment. The Birmingham Energy of funding: £12m for cryogenic energy storage and MORE EFFICIENT AND RELIABLE, 4.0 and other novel manufacturing approaches, ITEMA will scale up Institute’s strength comes, not only from the concentration of £1m for thermal energy storage, as part of a £15m LOWER CARBON AND MORE and modularise the production of technologies that will improve the expertise in specialised centres, but also the breadth of knowledge efficiency of thermal energy systems. -

Cryogenic Liquid Containers
Safetygram 27 Cryogenic liquid containers Cryogenic liquid containers, also referred to as liquid cylinders, are double-walled vacuum vessels with multilayer insulation in the annular space. They are designed for the reliable and economic transportation and storage of liquefied gases at cryogenic temperatures, typically colder than –130°F (–90°C). There are two primary advantages of a liquid container. The first is that it contains a large volume of gas at a relatively low pressure compared to a compressed gas cylinder. The second is that it provides a source of cryogenic liquids which can be easily handled. Cryogenic liquid containers are often incorrectly referred to as dewars. Dewars are open, nonpressurized vessels for holding cryogenic liquids. The cryogenic products normally found in liquid containers are liquid nitrogen (LIN), liquid argon (LAR), liquid oxygen (LOX), and liquid helium (LHE). Carbon dioxide and nitrous oxide are also available as refrigerated liquids in similar containers. Although these containers are well insulated, heat will continuously leak into the product, due to the extremely large temperature difference between the cryogenic liquid and the ambient environment. The heat leak will cause some vaporization to occur. Vaporized product, if not used, will collect in the vapor space above the liquid and build pressure. This is referred to as head pressure. The head pressure will build in the container and periodically vent via the pressure relief valve. Vaporization rates will vary and may be as low as 0.4% or as high as 3% of the container’s volume per day. This is a normal and safe function of the container. -

1 Office Safe Handling of Cryogenic Materials Guidelines
Office Safe Handling of Cryogenic Materials Guidelines Applies to: (examples; Faculty,Staff, Students, etc) Faculty , Staff , Students Policy Overview: Issued: 07-01-2018 Next Review Date: 07-19-2022 Frequency of Review: Annually Applies to St. Louis College of Pharmacy students, faculty, and staff working with cryogenic materials. Definitions: Term Definition Cryogenic Liquids Liquefied gases with a boiling point lower than -130°F (-90°C) at an absolute pressure of 14.7 psi (101.3 kPa). Boiling Point The temperature of a substance at which the vapor pressure of the liquid equals the pressure surrounding the liquid and the liquid changes into a vapor. Expansion Ratio The volume of a given amount of that substance in liquid form compared to the volume of the same amount of substance in gaseous form, at room temperature and normal atmospheric pressure OSHA Permissible Oxygen Any level between 19.5 percent and 23.5 percent oxygen. Any level Levels above or below this range will cause respiratory problems up to, and including, asphyxiation and death. Asphyxiation Loss of consciousness or death caused by impairing normal breathing, as by gas or other noxious agents; choke; suffocate; smother. Dewars Liquid Dewar flasks are non-pressurized, vacuum-jacketed vessels, similar to a Thermos bottle. Dewars are designed with either loose-fitting caps or pressure relief valves, that prevents air and moisture from entering, yet allows excess pressure to vent. Freezer Farm Refers to a collection of multiple freezers, housed in one location. Details: 1. Introduction a. The primary hazards of cryogenic liquids include both physical hazards such as fire, explosion, and pressure, but also health hazards such as chemical toxicity or severe frostbite and asphyxiation.