Cryogens and Dry Ice SOP
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Dry Ice Blasting
Dry Ice Blasting The Dry Ice Blasting method can reduce the overall time spent on sanding, scraping or scrub- bing with solvents, acids and other cleaning agents. With no secondary waste generation there is no need for disposal of hazardous waste. This saves time and money while increasing worker safety. Online Cleaning of Fin Dry Ice Blasting can safely and effectively clean contaminants that plug and foul fin fan heat Fan Heat Exchangers exchangers. Dry Ice Blasting can be performed while the system is online so no costly downtime is required. Cleaning will increase airflow which will lower approach temperatures and increase efficiency. The dry nature of the process will mitigate the potential for harming motors, bearings or instrumentation. Other Applications May The demand to keep the equipment running often leads to deferred cleaning and maintenance, Also Benefit reduced efficiency and in some cases, outages caused by flashover. Dry ice blast cleaning can provide a non-conductive cleaning process that may allow equipment to be cleaned in-place without cool down or disassembly. This includes generators, turbines, stators, rotors, compressors and boilers. Industrial cleaning applications exist in markets as diverse as food & beverage equipment clean-up, pharmaceutical production, aerospace surfaces & components, and foundry core- making machinery. A Leading Supplier of Working with Linde Services offers you product reliability from an industry leader in carbon Carbon Dioxide dioxide applications. This includes: → A focus on safety -

Liquid Argon
Safetygram 8 Liquid argon Liquid argon is tasteless, colorless, odorless, noncorrosive, nonflammable, and extremely cold. Belonging to the family of rare gases, argon is the most plentiful, making up approximately 1% of the earth’s atmosphere. It is monatomic and extremely inert, forming no known chemical compounds. Since argon is inert, special materials of construction are not required. However, materials of construction must be selected to withstand the low temperature of liquid argon. Vessels and piping should be designed to American Society of Mechanical Engineers (ASME) specifications or the Department of Transportation (DOT) codes for the pressures and temperatures involved. Although used more commonly in the gaseous state, argon is commonly stored and transported as a liquid, affording a more cost-effective way of providing product supply. Liquid argon is a cryogenic liquid. Cryogenic liquids are liquefied gases that have a normal boiling point below –130°F (–90°C). Liquid argon has a boiling point of –303°F (–186°C). The temperature difference between the product and the surrounding environment, even in winter, is substantial. Keeping this surrounding heat from the product requires special equipment to store and handle cryogenic liquids. A typical system consists of the following components: a cryogenic storage tank, one or more vaporizers, a pressure control system, and all of the piping required for fill, vaporization. The cryogenic tank is constructed, in principle, like a vacuum bottle. It is designed to keep heat away from the liquid that is contained in the inner vessel. Vaporizers convert the liquid argon to its gaseous state. A pressure control manifold controls the pressure at which the gas is fed to the process. -

Cryogenic Liquid Nitrogen Vehicles (ZEV's)
International Journal of Scientific and Research Publications, Volume 6, Issue 9, September 2016 562 ISSN 2250-3153 Cryogenic Liquid Nitrogen Vehicles (ZEV’S) K J Yogesh Department Of Mechanical Engineering, Jain Engineering College, Belagavi Abstract- As a result of widely increasing air pollution available zero emission vehicle (ZEV) meeting it's standards are throughout the world & vehicle emissions having a major the electrically recharged ones, however these vehicles are also contribution towards the same, it makes its very essential to not a great success in the society due to its own limitations like engineer or design an alternative to the present traditional initial cost, slow recharge, speeds etc. Lead acid & Ni-Cd gasoline vehicles. Liquid nitrogen fueled vehicles can act as an batteries are the past of major technologies in the electric excellent alternative for the same. Liquefied N2 at cryogenic vehicles. They exhibit specific energy in the range of 30-40 W- temperatures can replace conventional fuels in cryogenic heat hr/kg. Lead- acid batteries take hours to recharge & the major engines used as a propellant. The ambient temperature of the drawback of the batteries in all the cases is their replacement surrounding vaporizes the liquid form of N2 under pressure & periodically. This directly/indirectly increases the operating cost leads to the formation of compressed N2 gas. This gas actuates a when studied carefully & thereby not 100% acceptable. pneumatic motor. A combination of multiple reheat open Recent studies make it clear that the vehicles using liquid Rankine cycle & closed Brayton cycle are involved in the nitrogen as their means provide an excellent alternative before process to make use of liquid N2 as a non-polluting fuel. -

Capacity Enhancement of Indigenous Expansion Engine Based Helium Liquefier
IOP Conference Series: Materials Science and Engineering PAPER • OPEN ACCESS Capacity enhancement of indigenous expansion engine based helium liquefier To cite this article: R S Doohan et al 2017 IOP Conf. Ser.: Mater. Sci. Eng. 171 012011 View the article online for updates and enhancements. This content was downloaded from IP address 170.106.202.8 on 27/09/2021 at 00:16 ICECICMC IOP Publishing IOP Conf. Series: Materials Science and Engineering 171 (2017) 012011 doi:10.1088/1757-899X/171/1/012011 International Conference on Recent Trends in Physics 2016 (ICRTP2016) IOP Publishing Journal of Physics: Conference Series 755 (2016) 011001 doi:10.1088/1742-6596/755/1/011001 Capacity enhancement of indigenous expansion engine based helium liquefier R S Doohan1, P K Kush1, G Maheshwari2 1Raja Ramanna Centre for Advanced Technology, Indore, Madhya Pradesh India. 2Institute of Engineering and Technology, DAVV, Indore, Madhya Pradesh, India. E-mail: [email protected] Abstract. Development of technology and understanding for large capacity helium refrigeration and liquefaction at helium temperature is indispensable for coming-up projects. A new version of helium liquefier designed and built to provide approximately 35 liters of liquid helium per hour. The refrigeration capacity of this reciprocating type expansion engine machine has been increased from its predecessor version with continuous improvement and deficiency debugging. The helium liquefier has been built using components by local industries including cryogenic Aluminum plate fin heat exchangers. Two compressors with nearly identical capacity have been deployed for the operation of system. Together they consume about 110 kW of electric power. The system employs liquid Nitrogen precooling to enhance liquid Helium yield. -
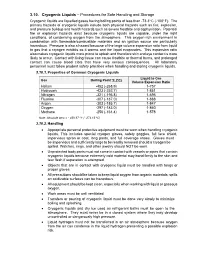
3.10. Cryogenic Liquids – Procedures for Safe Handling and Storage Cryogenic Liquids Are Liquefied Gases Having Boiling Points of Less Than -73.3O C (-100O F)
3.10. Cryogenic Liquids – Procedures for Safe Handling and Storage Cryogenic liquids are liquefied gases having boiling points of less than -73.3o C (-100o F). The primary hazards of cryogenic liquids include both physical hazards such as fire, explosion, and pressure buildup and health hazards such as severe frostbite and asphyxiation. Potential fire or explosion hazards exist because cryogenic liquids are capable, under the right conditions, of condensing oxygen from the atmosphere. This oxygen-rich environment in combination with flammable/combustible materials and an ignition source are particularly hazardous. Pressure is also a hazard because of the large volume expansion ratio from liquid to gas that a cryogen exhibits as it warms and the liquid evaporates. This expansion ratio also makes cryogenic liquids more prone to splash and therefore skin and eye contact is more likely to occur. Contact with living tissue can cause frostbite or thermal burns, and prolonged contact can cause blood clots that have very serious consequences. All laboratory personnel must follow prudent safety practices when handling and storing cryogenic liquids. 3.10.1. Properties of Common Cryogenic Liquids Liquid to Gas Gas Boiling Point oF (oC) Volume Expansion Ratio Helium -452 (-268.9) 1-757 Hydrogen -423 (-252.7) 1-851 Nitrogen -321 (-195.8) 1-696 Fluorine -307 (-187.0) 1-888 Argon -303 (-185.7) 1-847 Oxygen -297 (-183.0) 1-860 Methane -256 (-161.4) 1-578 Note: Absolute zero = - 459.67 oF (- 273.15 oC) 3.10.2. Handling Appropriate personal protective equipment must be worn when handling cryogenic liquids. -

NASA Spacecraft Observes Further Evidence of Dry Ice Gullies on Mars 11 July 2014, by Guy Webster
NASA spacecraft observes further evidence of dry ice gullies on Mars 11 July 2014, by Guy Webster "As recently as five years ago, I thought the gullies on Mars indicated activity of liquid water," said lead author Colin Dundas of the U.S. Geological Survey's Astrogeology Science Center in Flagstaff, Arizona. "We were able to get many more observations, and as we started to see more activity and pin down the timing of gully formation and change, we saw that the activity occurs in winter." Dundas and collaborators used the High Resolution Imaging Science Experiment (HiRISE) camera on MRO to examine gullies at 356 sites on Mars, beginning in 2006. Thirty-eight of the sites showed active gully formation, such as new channel segments and increased deposits at the downhill end of some gullies. Using dated before-and-after images, researchers determined the timing of this activity coincided with This pair of images covers one of the hundreds of sites seasonal carbon-dioxide frost and temperatures on Mars where researchers have repeatedly used the that would not have allowed for liquid water. High Resolution Imaging Science Experiment (HiRISE) camera on NASA's Mars Reconnaissance Orbiter to study changes in gullies on slopes. Credit: NASA/JPL- Frozen carbon dioxide, commonly called dry ice, Caltech/Univ. of Arizona does not exist naturally on Earth, but is plentiful on Mars. It has been linked to active processes on Mars such as carbon dioxide gas geysers and lines on sand dunes plowed by blocks of dry ice. One Repeated high-resolution observations made by mechanism by which carbon-dioxide frost might NASA's Mars Reconnaissance Orbiter (MRO) drive gully flows is by gas that is sublimating from indicate the gullies on Mars' surface are primarily the frost providing lubrication for dry material to formed by the seasonal freezing of carbon dioxide, flow. -

It's Just a Phase!
Bay Area Scientists in School Presentation Plan Lesson Name It’s just a phase!______________ Presenter(s) Kevin Metcalf, David Ojala, Melanie Drake, Carly Anderson, Hilda Buss, Lin Louie, Chris Jakobson California Standards Connection(s): 3rd Grade – Physical Science 3-PS-Matter has three states which can change when energy is added or removed. Next Generation Science Standards: 2nd Grade – Physical Science 2-PS1-1. Plan and conduct an investigation to describe and classify different kinds of materials by their observable properties. 2-PS1-4. Construct an argument with evidence that some changes caused by heating or cooling can be reversed and some cannot. Science & Engineering Practices Disciplinary Core Ideas Crosscutting Concepts Planning and carrying out PS1.A: Structure and Properties Patterns investigations to answer of Matter ・Patterns in the natural and questions or test solutions to ・Different kinds of matter exist human designed world can be problems in K–2 builds on prior and many of them can be observed. (2-PS1-1) experiences and progresses to either solid or liquid, simple investigations, based on depending on temperature. Cause and Effect fair tests, which provide data to Matter can be described and ・Events have causes that support explanations or design classified by its observable generate observable patterns. solutions. properties. (2-PS1-1) (2-PS1-4) ・Plan and conduct an ・Different properties are suited ・Simple tests can be designed to investigation collaboratively to to different purposes. (2-PS1- gather evidence to support or produce data to serve as the 2), (2-PS1-3) refute student ideas about basis for evidence to answer a ・A great variety of objects can causes. -

ACIDS and BASES Unique Properties of Dry Ice
ACIDS AND BASES Unique Properties of Dry Ice OVERVIEW: Students observe properties of dry ice. For example, whether dry ice can change the color of a basic solution that contains an indicator OBJECTIVE: To review basic principles of matter and how this relates to every day objects. Develop concepts and nature of acids and bases and relate this to common household items. GRADE LEVEL: 6-7 OHIO STANDARDS: PS7 Grade 7 Physical Science: The properties of matter are determined by the arrangement of atoms. TIME: 30-45 minutes VOCABULARY: acid, base, organic, inorganic, indicator, pH, hydrogen ion, molecule, CO2, dry ice MATERIALS: (per group of 5-6 students) *Block of dry ice *8 Beakers—600 ml tall with wide mouth *1 Graduated cylinder-10ml *1 stirring rod IMPORTANT: *Gloves.—cotton and latex Read all instructions before *Safety glasses proceeding *Indicator Solutions *pH paper *Balloons *1 pint Ammonia * 1 pint Vinegar DEVELOPED BY: Bob Maloney, Analytical Chemist, BP Chemicals INDICATOR SOLUTIONS: 100-250 ml of one or more of the following: *Grape juice concentrate *Cherry Juice *Beet Juice *Red Cabbage Juice VARIATION: 10-15 ml of one of the following: Thymolphthalein solution: (To prepare 100 ml of stock solution, dissolve 0.04g of thymolphthalein in 50 ml of 95% ethyl alcohol (ethanol) and dilute the resulting solution to 100ml with water. Alternatively, “Disappearing Ink” which is sold in toy stores can be used). Phenolphthalein solution (To prepare 10ml stock solution, dissolve 0.05g phenolphthalein in 50 ml of 95% ethyl alcohol and dilute the resulting solution to 100ml with water. Alternatively, crush two or three EX-Lax tablets and cover with rubbing alcohol and mix. -

Cryocoolers and Heat Exchangers Special Feature
SPECIAL FEATURE – CRYOCOOLERS AND HEAT EXCHANGERS SPECIAL FEATURE evolve from here? Whilst Brouwers “There is more need for path cleanliness. In focus... said that the company’s plans were cryogenic cold at a certain Awe confirmed that CAS has also confidential, he did say that it sees noticed a drastic upturn in demand possibilities in the market in terms of place, at a certain moment, for heat exchanger technology in the Cryocoolers and heat exchangers systems with a higher output compared at a certain time...” LNG sector, highlighting, “Natural with the output that its technology gas processors are morphing from By Rhea Healy can provide at the moment. “That markets, as well as customer bases in the successfully generating and capturing might be a combination of increasing LNG sector. these materials to rolling them out to the hether it’s heating media up the output of our existing technology Jeffery Awe, Marketing Director at marketplace on a massive scale. As the or cooling it down, time is of and systems, but it will also be in CAS, confirmed that the LNG sector is LNG and compressed natural gas (CNG) the essence in the industrial combination with the development of currently the most ‘vibrant’ in terms of industries continue to expand, we’d like gas industry. Companies and new systems. Ultimately, our main goal demand for the company’s technology, to parallel that growth trajectory in our Wengineers need gases and liquid gases to is to be recognised by our customers as along with its traditional industrial gas CAST-X line. -

Cryogenic Liquid Safety
Cryogenic Liquid Safety Hazards and Safe Handling of Cryogenic Liquids Cryogenic liquids have boiling points less than -73ºC (-100ºF). Liquid nitrogen, liquid oxygen and carbon dioxide are the most common cryogenic materials used in the laboratory. Hazards may include fire, explosion, embrittlement, pressure buildup, frostbite and asphyxiation. Many of the safety precautions observed for compressed gases also apply to cryogenic liquids. There are also additional hazards due to the unique properties of cryogenic liquids: • Extremely Low Temperatures: The cold boil-off vapor of cryogenic liquids rapidly freezes human tissue. Most metals become stronger upon exposure to cold temperatures, but materials, such as; carbon steel, plastics and rubber become brittle or fracture under stress at these temperatures. Proper material selection is important. Cold burns and frostbite caused by cryogenic liquids can result in extensive tissue damage. • Vaporization: All cryogenic liquids produce large volumes of gas when they vaporize. Liquid nitrogen will expand 696 times as it vaporizes. The expansion ratio of argon is 847:1, hydrogen is 851:1 and oxygen is 862:1. If these liquids vaporize in a sealed container, they can produce enormous pressures that could rupture the vessel. For this reason, pressurized cryogenic containers are usually protected with multiple pressure relief devices. • Vaporization of cryogenic liquids (except oxygen) in an enclosed area can cause asphyxiation! • Vaporization of liquid O2 can produce an oxygen-rich atmosphere, supporting and accelerating combustion of other materials. Vaporization of liquid hydrogen can form an extremely flammable mixture with air. Most cryogenic liquids are odorless, colorless, and tasteless when vaporized. When cryogenic liquids are exposed to the atmosphere, cold boil-off gases condense the moisture in the air, creating a highly visible fog. -

NASA Observations Point to 'Dry Ice' Snowfall on Mars 12 September 2012
NASA observations point to 'dry ice' snowfall on Mars 12 September 2012 "These are the first definitive detections of carbon- dioxide snow clouds," said the report's lead author, Paul Hayne of NASA's Jet Propulsion Laboratory in Pasadena, Calif. "We firmly establish the clouds are composed of carbon dioxide—flakes of Martian air—and they are thick enough to result in snowfall accumulation at the surface." The snowfalls occurred from clouds around the Red Planet's south pole in winter. The presence of carbon-dioxide ice in Mars' seasonal and residual southern polar caps has been known for decades. Also, NASA's Phoenix Lander mission in 2008 observed falling water-ice snow on northern Mars. Hayne and six co-authors analyzed data gained by looking at clouds straight overhead and sideways Carbon-Dioxide Snowfall on Mars. Observations by with the Mars Climate Sounder, one of six NASA's Mars Reconnaissance Orbiter have detected instruments on the Mars Reconnaissance Orbiter. carbon-dioxide snow clouds on Mars and evidence of carbon-dioxide snow falling to the surface. Deposits of This instrument records brightness in nine small particles of carbon-dioxide ice are formed by wavebands of visible and infrared light as a way to snowfall from carbon-dioxide clouds. This map shows examine particles and gases in the Martian the distribution of small-grain carbon-dioxide ice deposits atmosphere. The analysis was conducted while formed by snowfall over the south polar cap of Mars. It is Hayne was a post-doctoral fellow at the California based on infrared measurements by the Mars Climate Institute of Technology in Pasadena. -

Liquid Helium
Safetygram 22 Liquid helium Liquid helium is inert, colorless, odorless, noncorrosive, extremely cold, and nonflammable. Helium will not react with other elements or compounds under ordinary conditions. Since helium is noncorrosive, special materials of construction are not required. However, materials must be suitable for use at the extremely low temperatures of liquid helium. Vessels and piping must be selected and designed to withstand the pressure and temperatures involved and comply with applicable codes for transport and use. Manufacture Most of commercial helium is recovered from natural gas through a cryogenic separation process. Normally, helium is present in less than 1% by volume in natural gas. Helium is recovered, refined, and liquefied. Liquid helium is typically shipped from production sources to storage and transfill facilities. Tankers, ranging in size from 5,000 to 11,000 gallons, contain an annular space insulated with vacuum, nitrogen shielding, and multilayer insulation. This de- sign reduces heat leak and vaporization of liquid helium during transportation. Uses The extremely low temperature of liquid helium is utilized to maintain the superconducting properties of magnets in applications such as MRI, NMR spectroscopy, and particle physics research. The main application for gas- eous helium is for inert shielding gas in metal arc and laser welding. Helium provides a protective atmosphere in the production of reactive metals, such as titanium and zirconium. Gaseous helium is used as a coolant during the draw- ing of optical fibers, as a carrier gas for chromatography, and as a leak detection gas in a variety of industries. Being both lighter than air and nonflammable, helium is used to inflate balloons and airships.