Liquid Oxygen
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Liquid Argon
Safetygram 8 Liquid argon Liquid argon is tasteless, colorless, odorless, noncorrosive, nonflammable, and extremely cold. Belonging to the family of rare gases, argon is the most plentiful, making up approximately 1% of the earth’s atmosphere. It is monatomic and extremely inert, forming no known chemical compounds. Since argon is inert, special materials of construction are not required. However, materials of construction must be selected to withstand the low temperature of liquid argon. Vessels and piping should be designed to American Society of Mechanical Engineers (ASME) specifications or the Department of Transportation (DOT) codes for the pressures and temperatures involved. Although used more commonly in the gaseous state, argon is commonly stored and transported as a liquid, affording a more cost-effective way of providing product supply. Liquid argon is a cryogenic liquid. Cryogenic liquids are liquefied gases that have a normal boiling point below –130°F (–90°C). Liquid argon has a boiling point of –303°F (–186°C). The temperature difference between the product and the surrounding environment, even in winter, is substantial. Keeping this surrounding heat from the product requires special equipment to store and handle cryogenic liquids. A typical system consists of the following components: a cryogenic storage tank, one or more vaporizers, a pressure control system, and all of the piping required for fill, vaporization. The cryogenic tank is constructed, in principle, like a vacuum bottle. It is designed to keep heat away from the liquid that is contained in the inner vessel. Vaporizers convert the liquid argon to its gaseous state. A pressure control manifold controls the pressure at which the gas is fed to the process. -

Cryogenic Liquid Nitrogen Vehicles (ZEV's)
International Journal of Scientific and Research Publications, Volume 6, Issue 9, September 2016 562 ISSN 2250-3153 Cryogenic Liquid Nitrogen Vehicles (ZEV’S) K J Yogesh Department Of Mechanical Engineering, Jain Engineering College, Belagavi Abstract- As a result of widely increasing air pollution available zero emission vehicle (ZEV) meeting it's standards are throughout the world & vehicle emissions having a major the electrically recharged ones, however these vehicles are also contribution towards the same, it makes its very essential to not a great success in the society due to its own limitations like engineer or design an alternative to the present traditional initial cost, slow recharge, speeds etc. Lead acid & Ni-Cd gasoline vehicles. Liquid nitrogen fueled vehicles can act as an batteries are the past of major technologies in the electric excellent alternative for the same. Liquefied N2 at cryogenic vehicles. They exhibit specific energy in the range of 30-40 W- temperatures can replace conventional fuels in cryogenic heat hr/kg. Lead- acid batteries take hours to recharge & the major engines used as a propellant. The ambient temperature of the drawback of the batteries in all the cases is their replacement surrounding vaporizes the liquid form of N2 under pressure & periodically. This directly/indirectly increases the operating cost leads to the formation of compressed N2 gas. This gas actuates a when studied carefully & thereby not 100% acceptable. pneumatic motor. A combination of multiple reheat open Recent studies make it clear that the vehicles using liquid Rankine cycle & closed Brayton cycle are involved in the nitrogen as their means provide an excellent alternative before process to make use of liquid N2 as a non-polluting fuel. -

Capacity Enhancement of Indigenous Expansion Engine Based Helium Liquefier
IOP Conference Series: Materials Science and Engineering PAPER • OPEN ACCESS Capacity enhancement of indigenous expansion engine based helium liquefier To cite this article: R S Doohan et al 2017 IOP Conf. Ser.: Mater. Sci. Eng. 171 012011 View the article online for updates and enhancements. This content was downloaded from IP address 170.106.202.8 on 27/09/2021 at 00:16 ICECICMC IOP Publishing IOP Conf. Series: Materials Science and Engineering 171 (2017) 012011 doi:10.1088/1757-899X/171/1/012011 International Conference on Recent Trends in Physics 2016 (ICRTP2016) IOP Publishing Journal of Physics: Conference Series 755 (2016) 011001 doi:10.1088/1742-6596/755/1/011001 Capacity enhancement of indigenous expansion engine based helium liquefier R S Doohan1, P K Kush1, G Maheshwari2 1Raja Ramanna Centre for Advanced Technology, Indore, Madhya Pradesh India. 2Institute of Engineering and Technology, DAVV, Indore, Madhya Pradesh, India. E-mail: [email protected] Abstract. Development of technology and understanding for large capacity helium refrigeration and liquefaction at helium temperature is indispensable for coming-up projects. A new version of helium liquefier designed and built to provide approximately 35 liters of liquid helium per hour. The refrigeration capacity of this reciprocating type expansion engine machine has been increased from its predecessor version with continuous improvement and deficiency debugging. The helium liquefier has been built using components by local industries including cryogenic Aluminum plate fin heat exchangers. Two compressors with nearly identical capacity have been deployed for the operation of system. Together they consume about 110 kW of electric power. The system employs liquid Nitrogen precooling to enhance liquid Helium yield. -

Atlas V Cutaway Poster
ATLAS V Since 2002, Atlas V rockets have delivered vital national security, science and exploration, and commercial missions for customers across the globe including the U.S. Air Force, the National Reconnaissance Oice and NASA. 225 ft The spacecraft is encapsulated in either a 5-m (17.8-ft) or a 4-m (13.8-ft) diameter payload fairing (PLF). The 4-m-diameter PLF is a bisector (two-piece shell) fairing consisting of aluminum skin/stringer construction with vertical split-line longerons. The Atlas V 400 series oers three payload fairing options: the large (LPF, shown at left), the extended (EPF) and the extra extended (XPF). The 5-m PLF is a sandwich composite structure made with a vented aluminum-honeycomb core and graphite-epoxy face sheets. The bisector (two-piece shell) PLF encapsulates both the Centaur upper stage and the spacecraft, which separates using a debris-free pyrotechnic actuating 200 ft system. Payload clearance and vehicle structural stability are enhanced by the all-aluminum forward load reactor (FLR), which centers the PLF around the Centaur upper stage and shares payload shear loading. The Atlas V 500 series oers 1 three payload fairing options: the short (shown at left), medium 18 and long. 1 1 The Centaur upper stage is 3.1 m (10 ft) in diameter and 12.7 m (41.6 ft) long. Its propellant tanks are constructed of pressure-stabilized, corrosion-resistant stainless steel. Centaur is a liquid hydrogen/liquid oxygen-fueled vehicle. It uses a single RL10 engine producing 99.2 kN (22,300 lbf) of thrust. -

Phase Change: Titan’S Disappearing Lakes
Phase Change: Titan’s Disappearing Lakes Investigation Notebook NYC Edition © 2018 by The Regents of the University of California. All rights reserved. No part of this publication may be reproduced or transmitted in any form or by any means, electronic or mechanical, including photocopy, recording, or any information storage or retrieval system, without permission in writing from the publisher. Teachers purchasing this Investigation Notebook as part of a kit may reproduce the book herein in sufficient quantities for classroom use only and not for resale. These materials are based upon work partially supported by the National Science Foundation under grant numbers DRL-1119584, DRL-1417939, ESI-0242733, ESI-0628272, and ESI-0822119. The Federal Government has certain rights in this material. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation. These materials are based upon work partially supported by the Institute of Education Sciences, U.S. Department of Education, through Grant R305A130610 to The Regents of the University of California. The opinions expressed are those of the authors and do not represent views of the Institute or the U.S. Department of Education. Developed by the Learning Design Group at the University of California, Berkeley’s Lawrence Hall of Science. Amplify. 55 Washington Street, Suite 800 Brooklyn, NY 11201 1-800-823-1969 www.amplify.com Phase Change: Titan’s Disappearing Lakes -
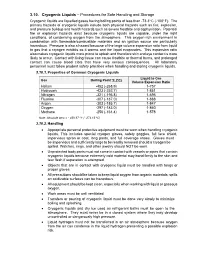
3.10. Cryogenic Liquids – Procedures for Safe Handling and Storage Cryogenic Liquids Are Liquefied Gases Having Boiling Points of Less Than -73.3O C (-100O F)
3.10. Cryogenic Liquids – Procedures for Safe Handling and Storage Cryogenic liquids are liquefied gases having boiling points of less than -73.3o C (-100o F). The primary hazards of cryogenic liquids include both physical hazards such as fire, explosion, and pressure buildup and health hazards such as severe frostbite and asphyxiation. Potential fire or explosion hazards exist because cryogenic liquids are capable, under the right conditions, of condensing oxygen from the atmosphere. This oxygen-rich environment in combination with flammable/combustible materials and an ignition source are particularly hazardous. Pressure is also a hazard because of the large volume expansion ratio from liquid to gas that a cryogen exhibits as it warms and the liquid evaporates. This expansion ratio also makes cryogenic liquids more prone to splash and therefore skin and eye contact is more likely to occur. Contact with living tissue can cause frostbite or thermal burns, and prolonged contact can cause blood clots that have very serious consequences. All laboratory personnel must follow prudent safety practices when handling and storing cryogenic liquids. 3.10.1. Properties of Common Cryogenic Liquids Liquid to Gas Gas Boiling Point oF (oC) Volume Expansion Ratio Helium -452 (-268.9) 1-757 Hydrogen -423 (-252.7) 1-851 Nitrogen -321 (-195.8) 1-696 Fluorine -307 (-187.0) 1-888 Argon -303 (-185.7) 1-847 Oxygen -297 (-183.0) 1-860 Methane -256 (-161.4) 1-578 Note: Absolute zero = - 459.67 oF (- 273.15 oC) 3.10.2. Handling Appropriate personal protective equipment must be worn when handling cryogenic liquids. -

Launcherone Success Opens New Space Access Gateway Guy Norris January 22, 2021
1/22/21 7:05 1/6 LauncherOne Success Opens New Space Access Gateway Guy Norris January 22, 2021 With San Nicolas Island far below, LauncherOne headed for polar orbit. Credit: Virgin Orbit Virgin Orbit had barely tweeted news of the successful Jan. 17 space debut of its LauncherOne vehicle on social media when new launch contracts began arriving in the company’s email inbox. A testament to the pent-up market demand for small-satellite launch capability, the speedy reaction to the long-awaited demonstration of the new space-access vehicle paves the way for multiple follow-on Virgin Orbit missions by year-end and a potential doubling of the rate in 2022. First successful privately developed air-launched, liquid-fueled rocket Payloads deployed for NASA’s Venture Class Launch Services program The glitch-free !ight of LauncherOne on its second demonstration test was a critical and much-welcomed milestone for the Long Beach, California-based company. Coming almost nine years a"er the air-launch concept was #rst unveiled by Virgin founder Richard Branson, and six years a"er the start of full-scale development, the !ight followed last May’s #rst demonstration mission, which ended abruptly when the rocket motor shut o$ a"er just 4 sec. 1/22/21 7:05 2/6 A"er an exhaustive analysis and modi#cations to beef up the oxidizer feed line at the heart of the #rst !ight failure, the path to the Launch Demo 2 test was then delayed until January 2021 by the COVID-19 pandemic. With the LauncherOne system now proven, design changes veri#ed and the #rst 10 small satellites placed in orbit, Virgin Orbit is already focusing on the next steps to ramp up its production and launch-cadence capabilities. -

Cryogenic Liquid Safety
Cryogenic Liquid Safety Hazards and Safe Handling of Cryogenic Liquids Cryogenic liquids have boiling points less than -73ºC (-100ºF). Liquid nitrogen, liquid oxygen and carbon dioxide are the most common cryogenic materials used in the laboratory. Hazards may include fire, explosion, embrittlement, pressure buildup, frostbite and asphyxiation. Many of the safety precautions observed for compressed gases also apply to cryogenic liquids. There are also additional hazards due to the unique properties of cryogenic liquids: • Extremely Low Temperatures: The cold boil-off vapor of cryogenic liquids rapidly freezes human tissue. Most metals become stronger upon exposure to cold temperatures, but materials, such as; carbon steel, plastics and rubber become brittle or fracture under stress at these temperatures. Proper material selection is important. Cold burns and frostbite caused by cryogenic liquids can result in extensive tissue damage. • Vaporization: All cryogenic liquids produce large volumes of gas when they vaporize. Liquid nitrogen will expand 696 times as it vaporizes. The expansion ratio of argon is 847:1, hydrogen is 851:1 and oxygen is 862:1. If these liquids vaporize in a sealed container, they can produce enormous pressures that could rupture the vessel. For this reason, pressurized cryogenic containers are usually protected with multiple pressure relief devices. • Vaporization of cryogenic liquids (except oxygen) in an enclosed area can cause asphyxiation! • Vaporization of liquid O2 can produce an oxygen-rich atmosphere, supporting and accelerating combustion of other materials. Vaporization of liquid hydrogen can form an extremely flammable mixture with air. Most cryogenic liquids are odorless, colorless, and tasteless when vaporized. When cryogenic liquids are exposed to the atmosphere, cold boil-off gases condense the moisture in the air, creating a highly visible fog. -

AEROSPACE ENGINEERING AE449 Senior Design Project I!1 Auburn University, Alabama
AEROSPACE ENGINEERING AE449 Senior Design Project I!1 Auburn University, Alabama FINAL STUDY REPORT FOR THE SPACE SHUTTLE II ADVANCED SPACE TRANSPORTATION SYSTEM Volume I: Executive Summary Submitted to: Dr. James O. Nichols Submitted by: James N. Adinaro Philip A. Benefield Shelby D. Johnson Lisa K. Knight Date Submitted: April 27, 1989 Table of Contents t . 1.0 ProjectSummary 1 2.0 Review 2 3.0 Proposed System Configuration 3 3.1 Changes in Preliminary Configuration 3 3.2 Wing 3 3.3 VerticalTail 4 3.4 Forward Fuselage 6 3.5 Mid Fuselage 6 3.6Aft Fuselage 8 3.7 Fuel and OxidizerTanks 8 3.8 Payload Bay and Payload Bay Doors 9 3.9Thrust Structure 12 3.10 Ascent Propulsion 12 3.11 Fuel/OxidizerFeed System 12 3.12 OrbitalManeuvering System/Reaction Control System 14 3.13 Landing Structures 14 • '" 4.0 Performance/Mission Analysis 15 4.1 Launch Event Schedule 15 4.2 Booster Launch/Landing Event Schedule 16 4.3 Orbital Event Schedule 17 4.4 Orbiter Landing Event Schedule 17 5.0 Stability and Control 19 6.0 Interface With Other Systems 21 7.0 Safety Analysis 22 7.1 Ascent Propulsion Failure Modes 24 7.2 Structural Failure Modes 24 7.3 Electronic Controls Failure Modes 25 8.0 Bibliography 27 9.0 Miscellaneous Figures 29 1.0 Project Summary This reportsummarizes an investigationintothe feasibilityof establishinga second generationspace transportationsystem. Incorporatingsuccessfulsystems from the Space Shuttle and technologicaladvances made sinceitsconception,the second generation shuttle presentedhere was designed tobe a lower-cost,more reliablesystem which would guarantee accessto space well intothe next century.A fullyreusable,all-liquidpropellant booster/orbitercombination using parallelburn was selectedas the base configuration. -

Liquid Helium
Safetygram 22 Liquid helium Liquid helium is inert, colorless, odorless, noncorrosive, extremely cold, and nonflammable. Helium will not react with other elements or compounds under ordinary conditions. Since helium is noncorrosive, special materials of construction are not required. However, materials must be suitable for use at the extremely low temperatures of liquid helium. Vessels and piping must be selected and designed to withstand the pressure and temperatures involved and comply with applicable codes for transport and use. Manufacture Most of commercial helium is recovered from natural gas through a cryogenic separation process. Normally, helium is present in less than 1% by volume in natural gas. Helium is recovered, refined, and liquefied. Liquid helium is typically shipped from production sources to storage and transfill facilities. Tankers, ranging in size from 5,000 to 11,000 gallons, contain an annular space insulated with vacuum, nitrogen shielding, and multilayer insulation. This de- sign reduces heat leak and vaporization of liquid helium during transportation. Uses The extremely low temperature of liquid helium is utilized to maintain the superconducting properties of magnets in applications such as MRI, NMR spectroscopy, and particle physics research. The main application for gas- eous helium is for inert shielding gas in metal arc and laser welding. Helium provides a protective atmosphere in the production of reactive metals, such as titanium and zirconium. Gaseous helium is used as a coolant during the draw- ing of optical fibers, as a carrier gas for chromatography, and as a leak detection gas in a variety of industries. Being both lighter than air and nonflammable, helium is used to inflate balloons and airships. -

Liquid Nitrogen Safety & Handling
Liquid Nitrogen Safety & Handling 1.0 Liquid Nitrogen & Cryogenic Liquids a. Liquid nitrogen, LN2, is colorless, odorless and tasteless. It is non-flammable, physiologically inert and non-toxic. It is an extremely cold liquid with a boiling point of - 196ºC = -320ºF. b. Cryogenic liquids are liquefied gases that are kept in their liquid state at extremely low temperatures. Cryogenic liquids have boiling points below -150°C (-238°F) (Carbon dioxide and nitrous oxide, which have slightly higher boiling points are sometimes included in this category). All cryogenic liquids are gases at normal temperatures and pressures. All Cryogenic liquids produce large amounts of gas when they vaporize. c. There a many applications of LN2 in a biomedical research facility, one of the most common being cryopreservation, the storage of cells at temperatures below -130 ºC, which is essential in order to preserve biological material unaltered. 2.0 Safety Hazards Associated with Handling LN2 a. The extremely low temperature of the liquid can cause severe frostbite or eye damage upon contact. Symptoms of frostbite include change in skin color to white or grayish yellow and the pain after contact with LN2 may quickly subside. Items in contact with liquid nitrogen become extremely cold. Touching these items may result in torn flesh. Many substances become brittle upon contact with liquid nitrogen and may shatter when cold (such as common glass and large solid plastics), sending pieces of the material flying. b. Upon vaporization it expands by a factor of 700; one liter of liquid nitrogen becomes 24.6 cubic feet of nitrogen gas. -

Mr. Soon-Young Park Korea Aerospace Research Institute (KARI), Korea, Republic Of, [email protected]
70th International Astronautical Congress 2019 Paper ID: 50565 IAF SPACE PROPULSION SYMPOSIUM (C4) Propulsion System (1) (1) Author: Mr. Soon-Young Park Korea Aerospace Research Institute (KARI), Korea, Republic of, [email protected] Dr. Yoonwan Moon Korea Aerospace Research Institute (KARI), Korea, Republic of, [email protected] Dr. Hwan-Seok CHOI Korea Aerospace Research Institute (KARI), Korea, Republic of, [email protected] Dr. Chang Ho Choi Korea Aerospace Research Institute (KARI), Korea, Republic of, [email protected] Dr. Sangyeop Han Korea Aerospace Research Institute (KARI), Korea, Republic of, [email protected] Dr. Yeoung-Min Han Korea Aerospace Research Institute (KARI), Korea, Republic of, [email protected] Dr. Jinhan Kim Korea Aerospace Research Institute (KARI), Korea, Republic of, [email protected] DEVELOPMENT STATUS OF BOOSTER STAGE LIQUID ROCKET ENGINE OF KSLV-II PROGRAM Abstract South Korea's indigenous three-staged liquid propellant rocket Korea Space Launch Vehicle-II (KSLV- II) also known as Nuri is now developing by Korea Aerospace Research Institute (KARI). KSLV-II planned to launch into space in 2021 at the NARO Space Center located the southern coast of Korea peninsular. Both the booster stage and the second stage of KSLV-II are propelled by the newly developing 75-tonf thrust level liquid-propellant rocket engines (KRE-075) and the third stage is powered by a 7-tonf thrust level rocket engine (KRE-007). KRE-075 fueled by kerosene and liquid oxygen which have some heritage of predecessor LV (KSR-III and KSLV-I). For the simplicity of its design and the parallel development logic to reduce the development schedule of the key components such as combustion chamber and turbopump, we adopted the reliable gas-generator cycle.