Pharmaceutics I صيدالنيات 1
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Shear Thickening in Concentrated Suspensions: Phenomenology
Shear thickening in concentrated suspensions: phenomenology, mechanisms, and relations to jamming Eric Brown School of Natural Sciences, University of California, Merced, CA 95343 Heinrich M. Jaeger James Franck Institute, The University of Chicago, Chicago, IL 60637 (Dated: July 22, 2013) Shear thickening is a type of non-Newtonian behavior in which the stress required to shear a fluid increases faster than linearly with shear rate. Many concentrated suspensions of particles exhibit an especially dramatic version, known as Discontinuous Shear Thickening (DST), in which the stress suddenly jumps with increasing shear rate and produces solid-like behavior. The best known example of such counter-intuitive response to applied stresses occurs in mixtures of cornstarch in water. Over the last several years, this shear-induced solid-like behavior together with a variety of other unusual fluid phenomena has generated considerable interest in the physics of densely packed suspensions. In this review, we discuss the common physical properties of systems exhibiting shear thickening, and different mechanisms and models proposed to describe it. We then suggest how these mechanisms may be related and generalized, and propose a general phase diagram for shear thickening systems. We also discuss how recent work has related the physics of shear thickening to that of granular materials and jammed systems. Since DST is described by models that require only simple generic interactions between particles, we outline the broader context of other concentrated many-particle systems such as foams and emulsions, and explain why DST is restricted to the parameter regime of hard-particle suspensions. Finally, we discuss some of the outstanding problems and emerging opportunities. -

Rheology in Pharmaceutical Formulations-A Perspective
evelo f D pin l o g a D Mastropietro et al., J Develop Drugs 2013, 2:2 n r r u u g o s J Journal of Developing Drugs DOI: 10.4172/2329-6631.1000108 ISSN: 2329-6631 Review Article Open Access Rheology in Pharmaceutical Formulations-A Perspective David J Mastropietro1, Rashel Nimroozi2 and Hossein Omidian1* 1Department of Pharmaceutical Sciences, College of Pharmacy, Nova Southeastern University, Fort Lauderdale, Florida, USA 2Westside Regional Medical Center, Pharmacy Department, Plantation, Florida, USA Abstract Medications produced as semi-solids type product such as creams, ointments and lotions are based on emulsion or suspension type systems consisting of two or more incompatible materials. In order to be manufactured, these dosage forms need specific flow properties so they can be placed into a container, remain stable over time, dispensed, handled and properly applied to the affected area by patients. Rheology is therefore crucially important as it will directly affect the way a drug is formulated and developed, the quality of the raw and finished product, the drug efficacy, the way a patient adheres to the prescribed drug, and the overall healthcare cost. It can be concluded that there are inherent and independent factors that affect the flow property of a medicated material during every stage of its manufacturing all the way to its use. Keywords: Pharmaceutical formulation; Rheology; Viscosity; dampened as the particles easily slide over one another to maintain a Suspensions; Rheology modifiers; Hydrophilic polymers steady viscosity, or Newtonian behavior. If stress is applied at a faster rate, the spherical particles slide faster over each other to maintain Introduction their history of viscosity. -

Dilatancy in the Flow and Fracture of Stretched Colloidal Suspensions
Dilatancy in the flow and fracture of stretched colloidal suspensions M.I. Smith School of Engineering, University of Edinburgh, Kings Buildings, Mayfield Rd, Edinburgh, EH9 3JL, UK School of Physics and Astronomy, University of Nottingham, University Park, Nottingham, NG7 2RD e-mail: [email protected] R. Besseling SUPA, School of Physics and Astronomy, University of Edinburgh, Kings Buildings, Mayfield Rd, Edinburgh EH9 3JZ e-mail: [email protected] M.E. Cates SUPA, School of Physics and Astronomy, University of Edinburgh, Kings Buildings, Mayfield Rd, Edinburgh, EH9 3JZ, UK e-mail: m.e.cates@ ed.ac.uk V. Bertola* School of Engineering, University of Edinburgh, Kings Buildings, Mayfield Rd, Edinburgh, EH9 3JL, UK e-mail: [email protected] contacting author* 1 Dilatancy in the flow and fracture of stretched colloidal suspensions. Concentrated particulate suspensions, commonplace in the pharmaceutical, cosmetic and food industries, display intriguing rheology. In particular, the dramatic increase in viscosity with strain rate (shear thickening and jamming) which is often observed at high volume fractions, is of strong practical and fundamental importance. Yet manufacture of these products and their subsequent dispensing often involves flow geometries substantially different from that of simple shear flow experiments. Here we show that the elongation and breakage of a filament of a colloidal fluid under tensile loading is closely related to the jamming transition seen in its shear rheology. However, the modified flow geometry reveals important additional effects. Using a model system with nearly hard-core interactions, we provide evidence of surprisingly strong viscoelasticity in such a colloidal fluid under tension. -
Non-Newtonian Fluids Flow Characteristic of Non-Newtonian Fluid
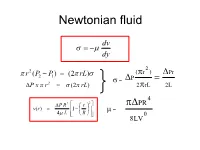
Newtonian fluid dv dy 2 r 2 (P P ) (2 rL) (r ) Pr 2 1 P 2 = P x r (2 rL) 2 rL 2L 2 4 P R2 r PR v(r) 1 4 L R = 8LV0 Definition of a Newtonian Fluid F du yx yx A dy For Newtonian behaviour (1) is proportional to and a plot passes through the origin; and (2) by definition the constant of proportionality, Newtonian dv dy 2 r 2 (P P ) (2 rL) (r ) Pr 2 1 P 2 = P x r (2 rL) 2 rL 2L 2 4 P R2 r PR v(r) 1 4 L R = 8LV0 Newtonian 2 From (r ) Pr d u = P 2rL 2L d y 4 and PR 8LQ = 0 4 8LV P = R = 8v/D 5 6 Non-Newtonian Fluids Flow Characteristic of Non-Newtonian Fluid • Fluids in which shear stress is not directly proportional to deformation rate are non-Newtonian flow: toothpaste and Lucite paint (Casson Plastic) (Bingham Plastic) Viscosity changes with shear rate. Apparent viscosity (a or ) is always defined by the relationship between shear stress and shear rate. Model Fitting - Shear Stress vs. Shear Rate Summary of Viscosity Models Newtonian n ( n 1) Pseudoplastic K n Dilatant K ( n 1) n Bingham y 1 1 1 1 Casson 2 2 2 2 0 c Herschel-Bulkley n y K or = shear stress, º = shear rate, a or = apparent viscosity m or K or K'= consistency index, n or n'= flow behavior index Herschel-Bulkley model (Herschel and Bulkley , 1926) n du m 0 dy Values of coefficients in Herschel-Bulkley fluid model Fluid m n Typical examples 0 Herschel-Bulkley >0 0<n< >0 Minced fish paste, raisin paste Newtonian >0 1 0 Water,fruit juice, honey, milk, vegetable oil Shear-thinning >0 0<n<1 0 Applesauce, banana puree, orange (pseudoplastic) juice concentrate Shear-thickening >0 1<n< 0 Some types of honey, 40 % raw corn starch solution Bingham Plastic >0 1 >0 Toothpaste, tomato paste Non-Newtonian Fluid Behaviour The flow curve (shear stress vs. -

Complex Fluids in Energy Dissipating Systems
applied sciences Review Complex Fluids in Energy Dissipating Systems Francisco J. Galindo-Rosales Centro de Estudos de Fenómenos de Transporte, Faculdade de Engenharia da Universidade do Porto, CP 4200-465 Porto, Portugal; [email protected] or [email protected]; Tel.: +351-925-107-116 Academic Editor: Fan-Gang Tseng Received: 19 May 2016; Accepted: 15 July 2016; Published: 25 July 2016 Abstract: The development of engineered systems for energy dissipation (or absorption) during impacts or vibrations is an increasing need in our society, mainly for human protection applications, but also for ensuring the right performance of different sort of devices, facilities or installations. In the last decade, new energy dissipating composites based on the use of certain complex fluids have flourished, due to their non-linear relationship between stress and strain rate depending on the flow/field configuration. This manuscript intends to review the different approaches reported in the literature, analyses the fundamental physics behind them and assess their pros and cons from the perspective of their practical applications. Keywords: complex fluids; electrorheological fluids; ferrofluids; magnetorheological fluids; electro-magneto-rheological fluids; shear thickening fluids; viscoelastic fluids; energy dissipating systems PACS: 82.70.-y; 83.10.-y; 83.60.-a; 83.80.-k 1. Introduction Preventing damage or discomfort resulting from any sort of external kinetic energy (impact or vibration) is an omnipresent problem in our society. On one hand, impacts and vibrations are responsible for several health problems. According to the European Injury Data Base (IDB), injuries due to accidents are killing one EU citizen every two minutes and disabling many more [1]. -

Non-Newtonian Fluid Math Dean Wheeler Brigham Young University May 2019
Non-Newtonian Fluid Math Dean Wheeler Brigham Young University May 2019 This document summarizes equations for computing pressure drop of a power-law fluid through a pipe. This is important to determine required pumping power for many fluids of practical importance, such as crude oil, melted plastics, and particle/liquid slurries. To simplify the analysis, I will assume the use of smooth and relatively long pipes (i.e. neglect pipe roughness and entrance effects). I further assume the reader has had exposure to principles taught in a college-level fluid mechanics course. Types of Fluids To begin, one must understand the difference between Newtonian and non-Newtonian fluids. There are many types of non-Newtonian fluids and this is a vast topic, which is studied under a branch of physics known as rheology. Rheology concerns itself with how materials (principally liquids, but also soft solids) flow under applied forces. Some non-Newtonian fluids exhibit time-dependent or viscoelastic behavior. This means their flow behavior depends on the history of what forces were applied to the fluid. Such fluids include thixotropic (shear-thinning over time) and rheopectic (shear-thickening over time) types. Viscoelastic fluids exhibit elastic or solid-like behavior when forces are first applied, and then transition to viscous flow under continuing force. These include egg whites, mucous, shampoo, and silly putty. We will not be analyzing any of these time-dependent fluids in this discussion. Time-independent or inelastic fluids flow with a constant rate when constant forces are applied to them. They are the focus of this discussion. -

Liquid Body Armor
Liquid Body Armor Organization: Children's Museum of Houston Contact person: Aaron Guerrero Contact information: [email protected] or 713‐535‐7226 General Description Type of program: Cart Demo Visitors will learn how nanotechnology is being used to create new types of protective fabrics. The classic experiment “Oobleck” is used to demonstrate how scientists are using similar techniques to recreate this phenomenon in flexible fabrics. Program Objectives Big idea: Nanotechnology can be used to create new protective fabrics with unique properties. Learning goals: As a result of participating in this program, visitors will be able to: 1. Explore and learn about the molecular behavior which gives Oobleck its unique properties. 2. Learn how nanotechnologists and material researchers are applying these properties to protective fabrics. NISE Network content map main ideas: [ ] 1. Nanometer‐sized things are very small, and often behave differently than larger things do. [ X ] 2. Scientists and engineers have formed the interdisciplinary field of nanotechnology by investigating properties and manipulating matter at the nanoscale. [ X ] 3. Nanoscience, nanotechnology, and nanoengineering lead to new knowledge and innovations that weren’t possible before. [ ] 4. Nanotechnologies have costs, risks, and benefits that affect our lives in ways we cannot always predict. 1 National Science Education Standards: 2. Physical Science [X] K‐4: Properties of objects and materials [ ] K‐4: Position and motion of objects [ ] K‐4: Light, heat, electricity, and magnetism [X] 5‐8: Properties and changes of properties in matter [X] 5‐8: Motions and forces [X] 5‐8: Transfer of energy [X] 9‐12: Structure of atoms [X] 9‐12: Structure and properties of matter [ ] 9‐12: Chemical reactions [X] 9‐12: Motions and force [ ] 9‐12: Conservation of energy and increase in disorder [X] 9‐12: Interactions of energy and matter 5. -

Numerical Simulation of the Flow of a Power Law Fluid in An
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Texas A&M University NUMERICAL SIMULATION OF THE FLOW OF A POWER LAW FLUID IN AN ELBOW BEND A Thesis by KARTHIK KANAKAMEDALA Submitted to the Office of Graduate Studies of Texas A&M University in partial fulfillment of the requirements for the degree of MASTER OF SCIENCE December 2009 Major Subject: Mechanical Engineering NUMERICAL SIMULATION OF THE FLOW OF A POWER LAW FLUID IN AN ELBOW BEND A Thesis by KARTHIK KANAKAMEDALA Submitted to the Office of Graduate Studies of Texas A&M University in partial fulfillment of the requirements for the degree of MASTER OF SCIENCE Approved by: Chair of Committee, K. R. Rajagopal Committee Members, N. K. Anand Hamn-Ching Chen Head of Department, Dennis O‟Neal December 2009 Major Subject: Mechanical Engineering iii ABSTRACT Numerical Simulation of the Flow of a Power Law Fluid in an Elbow Bend. (December 2009) Karthik Kanakamedala, B. Tech, National Institute of Technology Karnataka Chair of Advisory Committee: Dr. K. R. Rajagopal A numerical study of flow of power law fluid in an elbow bend has been carried out. The motivation behind this study is to analyze the velocity profiles, especially the pattern of the secondary flow of power law fluid in a bend as there are several important technological applications to which such a problem has relevance. This problem especially finds applications in the polymer processing industries and food industries where the fluid needs to be pumped through bent pipes. Hence, it is very important to study the secondary flow to determine the amount of power required to pump the fluid. -

The Thixotropy and Dilatancy of a Marine Soil
123 THE THIXOTROPY AND DILATANCY OF A MARINE SOIL By Garth Chapman, M.A. From the Department of Zoology, Queen Mary College, London (Text-figs. 1-6) CONTENTS PAGE Introduction and Historical 123 The Measurement of Resistance to Penetration 125 The Thixotropy and Dilatancy of Muddy Sand 127 Soil Hardness and Burrowing Speed of Arenicola 136 Conclusions 138 Summary 139 References 140 INTRODUCTION AND HISTORICAL In a"previous paper (Chapman & Newell, 1947) attention was called to the importance to their inhabitants of the thixotropic* and dilatant properties of marine soils. It will be recalled that the property of dilatancy, shown by the whitening of wet sand under the footstep, was first described by Osborne Reynolds (1885), and has subsequently been investigated chiefly by Freundlich and his collaborators (Freundlich, 1935; Freundlich & Jones, 1936; Freundlich & Roder, 1938). Reynolds considered that the close packing of particles in a liquid medium was altered by an applied force so that the interstitial volume was increased and more liquid was drawn from the periphery into the dilatant mixture. By these changes the dilatant mass becomes harder and more re- sistant to shear. Freundlich & Roder described the relation between thixotropy and dilatancy. They considered that thixotropy could be described as a re- duction in resistance with increased rate of shear as opposed to dilatancy, in which increased shearing force brings about an increased resistance. Thixo- * The term thixotropy was originally used by Peterfi and Freundlich for the isothermal reversible sol-gel transformation shown by some colloidal solutions, but it has now come to be applied more generally and is used to denote a system which shows a decrease in viscosity upon agitation or a decreased resistance to shear when the rate of shear increases. -

Rheology Theory and Applications
5KHRORJ\ 7KHRU\DQG$SSOLFDWLRQV TAINSTRUMENTS.COM &RXUVH2XWOLQH Basics in Rheology Theory Oscillation TA Rheometers Linear Viscoelasticity Instrumentation Setting up Oscillation Tests Choosing a Geometry Transient Testing Calibrations Applications of Rheology Flow Tests Polymers Viscosity Structured Fluids Setting up Flow Tests Advanced Accessories TAINSTRUMENTS.COM %DVLFVLQ5KHRORJ\7KHRU\ TAINSTRUMENTS.COM 5KHRORJ\$Q,QWURGXFWLRQ Rheology: The study of stress-deformation relationships TAINSTRUMENTS.COM 5KHRORJ\$Q,QWURGXFWLRQ Rheology is the science of flow and deformation of matter The word ‘Rheology’ was coined in the 1920s by Professor E C Bingham at Lafayette College in Indiana Flow is a special case of deformation The relationship between stress and deformation is a property of the material ୗ୲୰ୣୱୱ ౪౨౩౩ ୗ୦ୣୟ୰୰ୟ୲ୣ ൌ ౪౨ ൌ TAINSTRUMENTS.COM 6LPSOH6WHDG\6KHDU)ORZ Top plate Area = A x(t) Velocity = V0 Force = F y y Bottom Plate Velocity = 0 x F Shear Stress, Pascals σ = A x(t) σ γ = η = Shear Strain, % Viscosity, Pa⋅s y γ γ -1 γ = Shear Rate, sec t TAINSTRUMENTS.COM 7RUVLRQ)ORZLQ3DUDOOHO3ODWHV Ω r θ h Stress (σ) ߪൌ మ ൈM ഏೝయ r = plate radius h = distance between plates γ ߛൌೝ ൈθ M = torque (μN.m) Strain ( ) θ = Angular motor deflection (radians) Ω= Motor angular velocity (rad/s) ೝ Ω Strain rate (ߛሶ) ߛሶ ൌ ൈ TAINSTRUMENTS.COM 7$,QVWUXPHQWV5KHRPHWHUV TAINSTRUMENTS.COM 5RWDWLRQDO5KHRPHWHUVDW7$ ARES G2 DHR Controlled Strain Controlled Stress Dual Head Single Head SMT CMT TAINSTRUMENTS.COM 5RWDWLRQDO5KHRPHWHU'HVLJQV Dual head or SMT Single head or CMT Separate motor & transducer Combined motor & transducer Displacement Measured Transducer Sensor Torque (Stress) Measured Strain or Rotation Non-Contact Drag Cup Motor Applied Torque Sample (Stress) Applied Strain or Direct Drive ^ƚĂƚŝĐWůĂƚĞStatic Plate Rotation Motor Note: With computer feedback, DHR and AR can work in controlled strain/shear rate, and ARES can work in controlled stress. -

Shear Thickening Oscillation in a Dilatant Fluid
Journal of the Physical Society of Japan 80 (2011) 033801 LETTERS DOI: 10.1143/JPSJ.80.033801 Shear Thickening Oscillation in a Dilatant Fluid 1 Hiizu NAKANISHI and Namiko MITARAI Department of Physics, Kyushu University 33, Fukuoka 812-8581, Japan 1Niels Bohr Institute, University of Copenhagen, Blegdamsvej 17, DK-2100 Copenhagen ,Denmark (Received November 17, 2010; accepted January 11, 2011; published online February 25, 2011) By introducing a state variable, we construct a phenomenological fluid dynamical model of a dilatant fluid, i.e., a dense mixture of fluid and granules that shows severe shear thickening. We demonstrate that the fluid shows shear thickening oscillation, namely, the fluid flow oscillates owning to the coupling between the fluid dynamics and the internal dynamics of state. We also demonstrate that the jamming leads to a peculiar response to an external impact on the fluid. KEYWORDS: dilatant fluid, fluid dynamics, shear thickening, jamming, oscillatory instability z z A dense mixture of starch and water is an ideal material (a)Se (b) (c) z for demonstrating the shear thickening property of a non- h h g h Newtonian fluid. It may behave as liquid but immediately θ x solidifies upon sudden application of stress; thus, one can x x -h even run over a pool filled with this fluid. It is intriguing to Se see that it develops protrusions on its surface when sub- jected to strong vertical vibrations,1,2) and that it vibrates Fig. 1. Flow configurations with the coordinate system: (a) simple shear spontaneously when poured it out of a container. One source flow, (b) gravitational slope flow, and (c) impact-driven flow, where the upper wall is mobilized by a bullet impact. -

(12) United States Patent (10) Patent No.: US 6,701,529 B1 Rhoades Et Al
USOO6701529B1 (12) United States Patent (10) Patent No.: US 6,701,529 B1 Rhoades et al. (45) Date of Patent: Mar. 9, 2004 (54) SMART PADDING SYSTEM UTILIZING AN 5,506,290 A * 4/1996 Shapero ...................... 524/389 ENERGY ABSORBENT MEDUMAND 5,507866 A * 4/1996 Drew et al. .............. 106/287.1 ARTICLES MADE THEREFROM 5,527,204 A 6/1996 Rhoades ...................... 451/40 5,580,917 A 12/1996 Maciejewski et al. ...... 524/268 5,824,755 A * 10/1998 Hayashi et al. ............. 526/206 (75) Inventors. Lawrence JRhades, Pittsburgh, PA 5,854,143 A 12/1998 Schuster et al. ............ 442/135 (US); John M. Matechen, Irwin, PA 5869,1642Y- - - 2 A * 2/1999 Nickerson et al. ..... 297/.452.41 (US); Mark J. Rosner, Greensburg, PA 5.990.205 A * 11/1999 Cordova...................... 524/55 (US) 6,080,345 A 6/2000 Chalasani et al. .......... 264/109 6,347,411 B1 2/2002 Darling ......................... 2/272 (73) Assignee: Extrude Hone Corporation, Irwin, PA (US) FOREIGN PATENT DOCUMENTS (*) Notice: Subject to any disclaimer, the term of this FREP 2133O82O2984.49 E.1/1989 patent is extended or adjusted under 35 JP (1992)04-117903 4/1992 U.S.C. 154(b) by 0 days. JP (1992)04-117974 4/1992 WO WO88108860 11/1988 (21) Appl. No.: 09/489,513 OTHER PUBLICATIONS (22) Filed: Jan. 21, 2000 Article titled “Shear Thinning and Thickening”.* O O English language abstract to Russian Patent No. RU Related U.S. Application Data 2070903, published Dec. 27, 1996 (1 p.). (60) Provisional application No. 60/118,956, filed on Feb.