Rheology in Pharmaceutical Formulations-A Perspective
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Shear Thickening in Concentrated Suspensions: Phenomenology
Shear thickening in concentrated suspensions: phenomenology, mechanisms, and relations to jamming Eric Brown School of Natural Sciences, University of California, Merced, CA 95343 Heinrich M. Jaeger James Franck Institute, The University of Chicago, Chicago, IL 60637 (Dated: July 22, 2013) Shear thickening is a type of non-Newtonian behavior in which the stress required to shear a fluid increases faster than linearly with shear rate. Many concentrated suspensions of particles exhibit an especially dramatic version, known as Discontinuous Shear Thickening (DST), in which the stress suddenly jumps with increasing shear rate and produces solid-like behavior. The best known example of such counter-intuitive response to applied stresses occurs in mixtures of cornstarch in water. Over the last several years, this shear-induced solid-like behavior together with a variety of other unusual fluid phenomena has generated considerable interest in the physics of densely packed suspensions. In this review, we discuss the common physical properties of systems exhibiting shear thickening, and different mechanisms and models proposed to describe it. We then suggest how these mechanisms may be related and generalized, and propose a general phase diagram for shear thickening systems. We also discuss how recent work has related the physics of shear thickening to that of granular materials and jammed systems. Since DST is described by models that require only simple generic interactions between particles, we outline the broader context of other concentrated many-particle systems such as foams and emulsions, and explain why DST is restricted to the parameter regime of hard-particle suspensions. Finally, we discuss some of the outstanding problems and emerging opportunities. -

Pharmaceutics I صيدالنيات 1
Pharmaceutics I صيدﻻنيات 1 Unit 6 1 Rheology of suspensions • Rheology, the study of flow, addresses the viscosity characteristics of powders, fluids, and semisolids. • Materials are divided into two general categories, Newtonian and non-Newtonian, depending on their flow characteristics. • The unit of viscosity is the poise, the shearing force required to produce a velocity of 1 cm per second between two parallel planes of liquid, each 1 cm2 in area and separated by a distance of 1 cm. • The most convenient unit to use is the centipoise, or cP (equivalent to 0.01 poise). Newton’s law of flow • Newton law of flow relates parallel layers of liquid, with the bottom layer fixed, when a force is placed on the top layer, the top plane moves at constant velocity, each lower layer moves with a velocity directly proportional to its distance from the stationary bottom layer. • The velocity gradient, or rate of shear (dv/dr), is the difference of velocity dv between two planes of liquid separated by the distance dr. • The force (F´/A) applied to the top layer that is required to result in flow (rate of shear, G) is called the shearing stress (F). NEWTONIAN’S LOW OF FLOW Let us consider a block of liquid consisting of parallel plates of molecules as shown in the figure. The bottom layer is considered to be fixed in place. If the top plane of liquid is moved at constant velocity, each lower layer will move with a velocity directly proportional to its distance from the stationary bottom layer Representation of shearing force acting on a block of material ☻Rate of Shear dv/dr = G Is the velocity difference dv between two planes of liquid separated by an infinite distance dr. -

Dilatancy in the Flow and Fracture of Stretched Colloidal Suspensions
Dilatancy in the flow and fracture of stretched colloidal suspensions M.I. Smith School of Engineering, University of Edinburgh, Kings Buildings, Mayfield Rd, Edinburgh, EH9 3JL, UK School of Physics and Astronomy, University of Nottingham, University Park, Nottingham, NG7 2RD e-mail: [email protected] R. Besseling SUPA, School of Physics and Astronomy, University of Edinburgh, Kings Buildings, Mayfield Rd, Edinburgh EH9 3JZ e-mail: [email protected] M.E. Cates SUPA, School of Physics and Astronomy, University of Edinburgh, Kings Buildings, Mayfield Rd, Edinburgh, EH9 3JZ, UK e-mail: m.e.cates@ ed.ac.uk V. Bertola* School of Engineering, University of Edinburgh, Kings Buildings, Mayfield Rd, Edinburgh, EH9 3JL, UK e-mail: [email protected] contacting author* 1 Dilatancy in the flow and fracture of stretched colloidal suspensions. Concentrated particulate suspensions, commonplace in the pharmaceutical, cosmetic and food industries, display intriguing rheology. In particular, the dramatic increase in viscosity with strain rate (shear thickening and jamming) which is often observed at high volume fractions, is of strong practical and fundamental importance. Yet manufacture of these products and their subsequent dispensing often involves flow geometries substantially different from that of simple shear flow experiments. Here we show that the elongation and breakage of a filament of a colloidal fluid under tensile loading is closely related to the jamming transition seen in its shear rheology. However, the modified flow geometry reveals important additional effects. Using a model system with nearly hard-core interactions, we provide evidence of surprisingly strong viscoelasticity in such a colloidal fluid under tension. -

Methylcellulose, a Cellulose Derivative with Original Physical Properties and Extended Applications
Polymers 2015, 7, 777-803; doi:10.3390/polym7050777 OPEN ACCESS polymers ISSN 2073-4360 www.mdpi.com/journal/polymers Review Methylcellulose, a Cellulose Derivative with Original Physical Properties and Extended Applications Pauline L. Nasatto 1,2,*, Frédéric Pignon 2,3, Joana L. M. Silveira 1, Maria Eugênia R. Duarte 1, Miguel D. Noseda 1 and Marguerite Rinaudo 4 1 Departamento de Bioquímica e Biologia Molecular, Federal University of Paraná, P.O. Box 19046, CEP 81531-980, Curitiba, Paraná, Brazil; E-Mails: [email protected] (J.L.M.S.); [email protected] (M.E.R.D.); [email protected] (M.D.N.) 2 Laboratoire Rhéologie Procédés (LRP), University Grenoble Alpes, F-38000 Grenoble, France; E-Mail: [email protected] 3 Centre National de la Recherche Scientifique (CNRS), LRP, F-38000 Grenoble, France 4 Biomaterials Applications, 6, rue Lesdiguières, F-38000 Grenoble, France; E-Mail: [email protected] * Author to whom correspondence should be addressed; E-Mail: [email protected]; Tel.: +55-041-3361-1663; Fax: +55-041-3266-2041. Academic Editor: Antonio Pizzi Received: 5 February 2015 / Accepted: 15 April 2015 / Published: 24 April 2015 Abstract: This review covers the preparation, characterization, properties, and applications of methylcelluloses (MC). In particular, the influence of different chemical modifications of cellulose (under both heterogeneous and homogeneous conditions) is discussed in relation to the physical properties (solubility, gelation) of the methylcelluloses. The molecular weight (MW) obtained from the viscosity is presented together with the nuclear magnetic resonance (NMR) analysis required for the determination of the degree of methylation. The influence of the molecular weight on the main physical properties of methylcellulose in aqueous solution is analyzed. -

HPMC Capsules: Current Status and Future Prospects
J Pharm Pharmaceut Sci (www.cspsCanada.org) 13(3) 428 - 442, 2010 HPMC Capsules: Current Status and Future Prospects Moawia M. Al-Tabakha College of Pharmacy, Al Ain University of Science and Technology, Al-Ain, U.A.E. Received, July 13, 2010; Revised, September 20, 2010; Accepted, October 11, 2010; October 13, 2010 ABSTRACT Hydroxypropyl methylcellulose (HPMC) is employed for a wide variety of pharmaceutical and food preparations. Its applications as viscolizing agent (thickening agent), coating polymer, bioadhesive, in solid dispersion to enhance solubility, binder in the process of granulation and in modified release formulations have been well documented. One other notable use is in the production of capsule shells, replacing the animal derived gelatin in conventional two-piece capsules. The aim of this review is to systemically survey published literature on the HPMC use in capsule shells and resolve questions regarding their suitability as a replacement for hard gelatin capsules. Future refinements in the production and filling of HPMC capsule shells and improvement in their in vivo/in vitro dissolution would ensure their superiority over hard gelatin capsules. __________________________________________________________________________________________ INTRODUCTION over one side open body-piece forming closed cylindrical object (14). Capsules may offer better Hydroxypropyl methylcellulose (HPMC), now solid dosage form to tablets for drugs with low commonly known as hypromellose, is produced by compressibility, slow dissolution and bitter tasting. synthetic modification of the naturally occurring They are also used in clinical studies for blinding polymer cellulose and is considered safe for normal purpose. The administration of the capsules is consumption in humans (1). The material have been usually orally, but capsules for inhalation (15) such used and experimented as viscolizing agent i.e. -
Non-Newtonian Fluids Flow Characteristic of Non-Newtonian Fluid
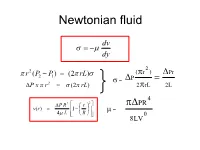
Newtonian fluid dv dy 2 r 2 (P P ) (2 rL) (r ) Pr 2 1 P 2 = P x r (2 rL) 2 rL 2L 2 4 P R2 r PR v(r) 1 4 L R = 8LV0 Definition of a Newtonian Fluid F du yx yx A dy For Newtonian behaviour (1) is proportional to and a plot passes through the origin; and (2) by definition the constant of proportionality, Newtonian dv dy 2 r 2 (P P ) (2 rL) (r ) Pr 2 1 P 2 = P x r (2 rL) 2 rL 2L 2 4 P R2 r PR v(r) 1 4 L R = 8LV0 Newtonian 2 From (r ) Pr d u = P 2rL 2L d y 4 and PR 8LQ = 0 4 8LV P = R = 8v/D 5 6 Non-Newtonian Fluids Flow Characteristic of Non-Newtonian Fluid • Fluids in which shear stress is not directly proportional to deformation rate are non-Newtonian flow: toothpaste and Lucite paint (Casson Plastic) (Bingham Plastic) Viscosity changes with shear rate. Apparent viscosity (a or ) is always defined by the relationship between shear stress and shear rate. Model Fitting - Shear Stress vs. Shear Rate Summary of Viscosity Models Newtonian n ( n 1) Pseudoplastic K n Dilatant K ( n 1) n Bingham y 1 1 1 1 Casson 2 2 2 2 0 c Herschel-Bulkley n y K or = shear stress, º = shear rate, a or = apparent viscosity m or K or K'= consistency index, n or n'= flow behavior index Herschel-Bulkley model (Herschel and Bulkley , 1926) n du m 0 dy Values of coefficients in Herschel-Bulkley fluid model Fluid m n Typical examples 0 Herschel-Bulkley >0 0<n< >0 Minced fish paste, raisin paste Newtonian >0 1 0 Water,fruit juice, honey, milk, vegetable oil Shear-thinning >0 0<n<1 0 Applesauce, banana puree, orange (pseudoplastic) juice concentrate Shear-thickening >0 1<n< 0 Some types of honey, 40 % raw corn starch solution Bingham Plastic >0 1 >0 Toothpaste, tomato paste Non-Newtonian Fluid Behaviour The flow curve (shear stress vs. -

Hydroxypropyl Methylcellulose E15: a Hydrophilic Polymer for Fabrication of Orodispersible Film Using Syringe Extrusion 3D Printer
polymers Article Hydroxypropyl Methylcellulose E15: A Hydrophilic Polymer for Fabrication of Orodispersible Film Using Syringe Extrusion 3D Printer Pattaraporn Panraksa 1 , Suruk Udomsom 2 , Pornchai Rachtanapun 3 , Chuda Chittasupho 1,4, Warintorn Ruksiriwanich 1,4 and Pensak Jantrawut 1,4,* 1 Department of Pharmaceutical Sciences, Faculty of Pharmacy, Chiang Mai University, Chiang Mai 50200, Thailand; [email protected] (P.P.); [email protected] (C.C.); [email protected] (W.R.) 2 Biomedical Engineering Institute, Chiang Mai University, Chiang Mai 50200, Thailand; [email protected] 3 Division of Packaging Technology, School of Agro-Industry, Faculty of Agro-Industry, Chiang Mai University, Chiang Mai 50100, Thailand; [email protected] 4 Cluster of Research and Development of Pharmaceutical and Natural Products Innovation for Human or Animal, Chiang Mai University, Chiang Mai 50200, Thailand * Correspondence: [email protected] or [email protected]; Tel.: +66-53944309 Received: 29 October 2020; Accepted: 11 November 2020; Published: 12 November 2020 Abstract: Extrusion-based 3D printing technology is a relatively new technique that has a potential for fabricating pharmaceutical products in various dosage forms. It offers many advantages over conventional manufacturing methods, including more accurate drug dosing, which is especially important for the drugs that require exact tailoring (e.g., narrow therapeutic index drugs). In this work, we have successfully fabricated phenytoin-loaded orodispersible films (ODFs) through a syringe extrusion 3D printing technique. Two different grades of hydroxypropyl methylcellulose (HPMC E5 and HPMC E15) were used as the film-forming polymers, and glycerin and propylene glycol were used as plasticizers. -

Complex Fluids in Energy Dissipating Systems
applied sciences Review Complex Fluids in Energy Dissipating Systems Francisco J. Galindo-Rosales Centro de Estudos de Fenómenos de Transporte, Faculdade de Engenharia da Universidade do Porto, CP 4200-465 Porto, Portugal; [email protected] or [email protected]; Tel.: +351-925-107-116 Academic Editor: Fan-Gang Tseng Received: 19 May 2016; Accepted: 15 July 2016; Published: 25 July 2016 Abstract: The development of engineered systems for energy dissipation (or absorption) during impacts or vibrations is an increasing need in our society, mainly for human protection applications, but also for ensuring the right performance of different sort of devices, facilities or installations. In the last decade, new energy dissipating composites based on the use of certain complex fluids have flourished, due to their non-linear relationship between stress and strain rate depending on the flow/field configuration. This manuscript intends to review the different approaches reported in the literature, analyses the fundamental physics behind them and assess their pros and cons from the perspective of their practical applications. Keywords: complex fluids; electrorheological fluids; ferrofluids; magnetorheological fluids; electro-magneto-rheological fluids; shear thickening fluids; viscoelastic fluids; energy dissipating systems PACS: 82.70.-y; 83.10.-y; 83.60.-a; 83.80.-k 1. Introduction Preventing damage or discomfort resulting from any sort of external kinetic energy (impact or vibration) is an omnipresent problem in our society. On one hand, impacts and vibrations are responsible for several health problems. According to the European Injury Data Base (IDB), injuries due to accidents are killing one EU citizen every two minutes and disabling many more [1]. -

Hydroxypropyl Methylcellulose
Hydroxypropyl Methylcellulose Processing 1 Executive Summary 2 3 Hydroxypropyl methylcellulose (HPMC) was petitioned as an ingredient of hard capsules used for encapsulating 4 powdered herbs. This use is petitioned as an alternative to gelatin (animal based) capsules. HPMC has many other 5 uses as an emulsifier, thickening agent, stabilizer, gellant, and suspending agent. 6 7 HPMC is a cellulose ether, derived from alkali treated cellulose that is reacted with methyl chloride and propylene 8 oxide. The NOSB approved powdered cellulose, a less processed material usually derived from wood pulp fiber, for 9 use as a filtering aid and anti-caking agent in October of 2001. 10 11 All reviewers found that HPMC is synthetic and nonagricultural, but were not in agreement about its use in organic 12 production. Two reviewers felt that due to the use of synthetic hazardous materials to produce HPMC, it is not 13 compatible with use in organic products, and that alternatives can be developed. Another reviewer recommended that 14 HPMC should be allowed, and restricted for use only in non-gelatin hard capsules, finding a lack of alternatives for 15 this use. Two reviewers supported use in products labeled “made with organic (specified ingredients),” while one 16 believes it should be prohibited for all uses 17 18 19 Summary of TAP Reviewer Analysis1 20 21 95% organic Synthetic / Non-Synthetic: Allowed or Prohibited: Suggested Annotation: Synthetic (3) Allowed (1) In hard non-gelatin capsules Non-synthetic (0) Prohibited (2) 22 23 Made with organic -

Non-Newtonian Fluid Math Dean Wheeler Brigham Young University May 2019
Non-Newtonian Fluid Math Dean Wheeler Brigham Young University May 2019 This document summarizes equations for computing pressure drop of a power-law fluid through a pipe. This is important to determine required pumping power for many fluids of practical importance, such as crude oil, melted plastics, and particle/liquid slurries. To simplify the analysis, I will assume the use of smooth and relatively long pipes (i.e. neglect pipe roughness and entrance effects). I further assume the reader has had exposure to principles taught in a college-level fluid mechanics course. Types of Fluids To begin, one must understand the difference between Newtonian and non-Newtonian fluids. There are many types of non-Newtonian fluids and this is a vast topic, which is studied under a branch of physics known as rheology. Rheology concerns itself with how materials (principally liquids, but also soft solids) flow under applied forces. Some non-Newtonian fluids exhibit time-dependent or viscoelastic behavior. This means their flow behavior depends on the history of what forces were applied to the fluid. Such fluids include thixotropic (shear-thinning over time) and rheopectic (shear-thickening over time) types. Viscoelastic fluids exhibit elastic or solid-like behavior when forces are first applied, and then transition to viscous flow under continuing force. These include egg whites, mucous, shampoo, and silly putty. We will not be analyzing any of these time-dependent fluids in this discussion. Time-independent or inelastic fluids flow with a constant rate when constant forces are applied to them. They are the focus of this discussion. -

Liquid Body Armor
Liquid Body Armor Organization: Children's Museum of Houston Contact person: Aaron Guerrero Contact information: [email protected] or 713‐535‐7226 General Description Type of program: Cart Demo Visitors will learn how nanotechnology is being used to create new types of protective fabrics. The classic experiment “Oobleck” is used to demonstrate how scientists are using similar techniques to recreate this phenomenon in flexible fabrics. Program Objectives Big idea: Nanotechnology can be used to create new protective fabrics with unique properties. Learning goals: As a result of participating in this program, visitors will be able to: 1. Explore and learn about the molecular behavior which gives Oobleck its unique properties. 2. Learn how nanotechnologists and material researchers are applying these properties to protective fabrics. NISE Network content map main ideas: [ ] 1. Nanometer‐sized things are very small, and often behave differently than larger things do. [ X ] 2. Scientists and engineers have formed the interdisciplinary field of nanotechnology by investigating properties and manipulating matter at the nanoscale. [ X ] 3. Nanoscience, nanotechnology, and nanoengineering lead to new knowledge and innovations that weren’t possible before. [ ] 4. Nanotechnologies have costs, risks, and benefits that affect our lives in ways we cannot always predict. 1 National Science Education Standards: 2. Physical Science [X] K‐4: Properties of objects and materials [ ] K‐4: Position and motion of objects [ ] K‐4: Light, heat, electricity, and magnetism [X] 5‐8: Properties and changes of properties in matter [X] 5‐8: Motions and forces [X] 5‐8: Transfer of energy [X] 9‐12: Structure of atoms [X] 9‐12: Structure and properties of matter [ ] 9‐12: Chemical reactions [X] 9‐12: Motions and force [ ] 9‐12: Conservation of energy and increase in disorder [X] 9‐12: Interactions of energy and matter 5. -

Numerical Simulation of the Flow of a Power Law Fluid in An
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Texas A&M University NUMERICAL SIMULATION OF THE FLOW OF A POWER LAW FLUID IN AN ELBOW BEND A Thesis by KARTHIK KANAKAMEDALA Submitted to the Office of Graduate Studies of Texas A&M University in partial fulfillment of the requirements for the degree of MASTER OF SCIENCE December 2009 Major Subject: Mechanical Engineering NUMERICAL SIMULATION OF THE FLOW OF A POWER LAW FLUID IN AN ELBOW BEND A Thesis by KARTHIK KANAKAMEDALA Submitted to the Office of Graduate Studies of Texas A&M University in partial fulfillment of the requirements for the degree of MASTER OF SCIENCE Approved by: Chair of Committee, K. R. Rajagopal Committee Members, N. K. Anand Hamn-Ching Chen Head of Department, Dennis O‟Neal December 2009 Major Subject: Mechanical Engineering iii ABSTRACT Numerical Simulation of the Flow of a Power Law Fluid in an Elbow Bend. (December 2009) Karthik Kanakamedala, B. Tech, National Institute of Technology Karnataka Chair of Advisory Committee: Dr. K. R. Rajagopal A numerical study of flow of power law fluid in an elbow bend has been carried out. The motivation behind this study is to analyze the velocity profiles, especially the pattern of the secondary flow of power law fluid in a bend as there are several important technological applications to which such a problem has relevance. This problem especially finds applications in the polymer processing industries and food industries where the fluid needs to be pumped through bent pipes. Hence, it is very important to study the secondary flow to determine the amount of power required to pump the fluid.