WO 2016/172658 A2 27 October 2016 (27.10.2016) P O P C T
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Meta Gene 2 (2014) 746–760
Meta Gene 2 (2014) 746–760 Contents lists available at ScienceDirect Meta Gene Characterization of the bovine gene LIPE and possible influence on fatty acid composition of meat Daniel Estanislao Goszczynski a,c, Juliana Papaleo Mazzucco b, María Verónica Ripoli a, Edgardo Leopoldo Villarreal b, Andrés Rogberg-Muñoz a, Carlos Alberto Mezzadra b, Lilia Magdalena Melucci b,⁎, Guillermo Giovambattista a,⁎⁎ a IGEVET, CCT LA PLATA CONICET, FCV, UNLP, La Plata B1900AVW, CC 296, Argentina b Unidad Integrada INTA Balcarce — FCA, UNMdP, Argentina c Fellow of the Consejo Nacional de Investigaciones Científicas y Tecnológicas (CONICET), Argentina article info abstract Article history: LIPE is an intracellular neutral lipase, which is capable of hydrolyzing a va- Received 10 June 2014 riety of esters and plays a key role in the mobilization of fatty acids from Revised 26 August 2014 diacylglycerols. The objectives of this study were to characterize the Accepted 3 September 2014 genetic polymorphism of bovine LIPE gene and to evaluate the possible Available online 16 October 2014 association between three SNPs in the coding regions of this gene with the fatty acid composition of meat in a cattle population. Forty-three unre- Keywords: lated animals from different cattle breeds were re-sequenced and 21 SNPs LIPE fi Polymorphism were detected over approximately 2600 bp, ve of these SNPs were novel. Bovine Three SNPs were selected, on the basis of evolutionary conservation, to Lipid content perform validation and association studies in a crossbred cattle popula- tion. Our results may suggest a possible association of SNP1 with contents of oleic acid and total monounsaturated fatty acids (p b 0.01), and SNP2 and SNP3 with Heneicosylic acid content (p b 0.01), may be helpful to improve the quality of meat and improve health. -

(12) United States Patent (10) Patent No.: US 9,375.433 B2 Dilly Et Al
US009375433B2 (12) United States Patent (10) Patent No.: US 9,375.433 B2 Dilly et al. (45) Date of Patent: *Jun. 28, 2016 (54) MODULATORS OF ANDROGENSYNTHESIS (52) U.S. Cl. CPC ............. A6 IK3I/519 (2013.01); A61 K3I/201 (71) Applicant: Tangent Reprofiling Limited, London (2013.01); A61 K3I/202 (2013.01); A61 K (GB) 31/454 (2013.01); A61K 45/06 (2013.01) (72) Inventors: Suzanne Dilly, Oxfordshire (GB); (58) Field of Classification Search Gregory Stoloff, London (GB); Paul USPC .................................. 514/258,378,379, 560 Taylor, London (GB) See application file for complete search history. (73) Assignee: Tangent Reprofiling Limited, London (56) References Cited (GB) U.S. PATENT DOCUMENTS (*) Notice: Subject to any disclaimer, the term of this 5,364,866 A * 1 1/1994 Strupczewski.......... CO7C 45/45 patent is extended or adjusted under 35 514,254.04 U.S.C. 154(b) by 0 days. 5,494.908 A * 2/1996 O’Malley ............. CO7D 261/20 514,228.2 This patent is Subject to a terminal dis 5,776,963 A * 7/1998 Strupczewski.......... CO7C 45/45 claimer. 514,217 6,977.271 B1* 12/2005 Ip ........................... A61K 31, 20 (21) Appl. No.: 14/708,052 514,560 OTHER PUBLICATIONS (22) Filed: May 8, 2015 Calabresi and Chabner (Goodman & Gilman's The Pharmacological (65) Prior Publication Data Basis of Therapeutics, 10th ed., 2001).* US 2015/O238491 A1 Aug. 27, 2015 (Cecil's Textbook of Medicine pp. 1060-1074 published 2000).* Stedman's Medical Dictionary (21st Edition, Published 2000).* Okamoto et al (Journal of Pain and Symptom Management vol. -

RAP-MD-05 GLYX13-C-203 Protocol Amendment 3 Final-R
NCT02192099 Study ID: GLYX13‐C‐203 Title: Open Label Extension for Subjects with Inadequate/Partial Response to Antidepressants during the Current Episode of Major Depressive Disorder Previously Treated with Rapastinel (GLYX‐13) (Extension of GLYX13‐C‐202, NCT01684163) Protocol Amendment 3 Date: 23 Nov 2016 Clinical Study Protocol Protocol Number: GLYX13-C-203 Study Drug: Rapastinel (GLYX-13) Injection (rapastinel Injection) FDA ID: 107,974 Clinicaltrials.gov: Title: Open Label Extension for Subjects with Inadequate/Partial Response to Antidepressants during the Current Episode of Major Depressive Disorder Previously Treated with Rapastinel (GLYX-13) (Extension of GLYX13-C-202, NCT01684163) Study Phase: 2 Sponsor: Naurex, Inc, an affiliate of Allergan, plc. Date: Amendment 3 dated 23 November 2016 Replaces Amendment 2 dated 20 January 2015 This document is a confidential communication from Naurex, Inc, an affiliate of Allergan, plc.. Acceptance of this document constitutes an agreement by the recipient(s) that no information contained herein will be published or disclosed without prior written approval from Allergan, plc., except that this document may be disclosed to appropriate Institutional Review Boards (IRBs) under the condition that they are also required to maintain confidentiality. Naurex, Inc, an affiliate of Allergan, plc. Protocol GLYX13-C-203 INVESTIGATOR SIGNATURE PAGE The signature of the investigator below constitutes his/her approval of this protocol and provides the necessary assurances that this study will be conducted according to all stipulations of the protocol as specified in both the clinical and administrative sections, including all statements regarding confidentiality. This trial will be conducted in compliance with the protocol and all applicable regulatory requirements, in accordance with Good Clinical Practices (GCPs), including International Conference on Harmonization (ICH) Guidelines, and in general conformity with the most recent version of the Declaration of Helsinki. -

WO 2017/074902 Al 4 May 20 17 (04.05.2017) W P O P C T
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2017/074902 Al 4 May 20 17 (04.05.2017) W P O P C T (51) International Patent Classification: AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, A61K 8/37 (2006.01) A61Q 19/00 (2006.01) BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DJ, DK, DM, A61K 31/215 (2006.01) DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, HN, HR, HU, ID, IL, IN, IR, IS, JP, KE, KG, KN, KP, KR, (21) International Application Number: KW, KZ, LA, LC, LK, LR, LS, LU, LY, MA, MD, ME, PCT/US2016/058591 MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, (22) International Filing Date: OM, PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SA, 25 October 2016 (25.10.201 6) SC, SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, TN, TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, (25) Filing Language: English ZW. (26) Publication Language: English (84) Designated States (unless otherwise indicated, for every (30) Priority Data: kind of regional protection available): ARIPO (BW, GH, 62/247,803 29 October 20 15 (29. 10.20 15) US GM, KE, LR, LS, MW, MZ, NA, RW, SD, SL, ST, SZ, TZ, UG, ZM, ZW), Eurasian (AM, AZ, BY, KG, KZ, RU, (71) Applicant: GLAXOSMITHKLINE CONSUMER TJ, TM), European (AL, AT, BE, BG, CH, CY, CZ, DE, HEALTHCARE HOLDINGS (US) LLC [US/US]; 271 1 DK, EE, ES, FI, FR, GB, GR, HR, HU, IE, IS, IT, LT, LU, Centerville Road, Suite 400, Wilmington, DE 19808 (US). -

Modeling the Effect of Heat Treatment on Fatty Acid Composition in Home-Made Olive Oil Preparations
Open Life Sciences 2020; 15: 606–618 Research Article Dani Dordevic, Ivan Kushkevych*, Simona Jancikova, Sanja Cavar Zeljkovic, Michal Zdarsky, Lucia Hodulova Modeling the effect of heat treatment on fatty acid composition in home-made olive oil preparations https://doi.org/10.1515/biol-2020-0064 refined olive oil in PUFAs, though a heating temperature received May 09, 2020; accepted May 25, 2020 of 220°C resulted in similar decrease in MUFAs and fi Abstract: The aim of this study was to simulate olive oil PUFAs, in both extra virgin and re ned olive oil samples. ff fi use and to monitor changes in the profile of fatty acids in The study showed di erences in fatty acid pro les that home-made preparations using olive oil, which involve can occur during the culinary heating of olive oil. repeated heat treatment cycles. The material used in the Furthermore, the study indicated that culinary heating experiment consisted of extra virgin and refined olive oil of extra virgin olive oil produced results similar to those fi samples. Fatty acid profiles of olive oil samples were of the re ned olive oil heating at a lower temperature monitored after each heating cycle (10 min). The out- below 180°C. comes showed that cycles of heat treatment cause Keywords: virgin olive oil, refined olive oil, saturated significant (p < 0.05) differences in the fatty acid profile fatty acids, monounsaturated fatty acids, polyunsatu- of olive oil. A similar trend of differences (p < 0.05) was rated fatty acids, cross-correlation analysis found between fatty acid profiles in extra virgin and refined olive oils. -

Improvement of Lipid Production from an Oil-Producing Filamentous Fungus, Penicillium Brevicompactum NRC 829, Through Central Composite Statistical Design
Ann Microbiol (2017) 67:601–613 DOI 10.1007/s13213-017-1287-x ORIGINAL ARTICLE Improvement of lipid production from an oil-producing filamentous fungus, Penicillium brevicompactum NRC 829, through central composite statistical design Thanaa H. Ali1 & Mamdouh S. El-Gamal2 & Dina H. El-Ghonemy1 & Ghada E. Awad3 & Amir E. Tantawy1 Received: 12 March 2017 /Accepted: 13 July 2017 /Published online: 7 August 2017 # Springer-Verlag GmbH Germany and the University of Milan 2017 Abstract In the present study, 13 filamentous fungi were commercial development for the production of LA by fer- screened for their lipid production and an oleaginous fun- mentation using cheap raw material. gus, Penicillium brevicompactum NRC 829, was found to be the highest lipid producer. Screening of various agro- Keywords Linoleic acid . Penicillium brevicompactum NRC industrial residues was performed and sunflower oil cake 829 . Response surface methodology . Unsaturated fatty acids proved to be the best substrate for lipid production. A central composite design was employed to investigate the optimum concentrations of the most significant medi- Introduction um components required to improve the lipid production by P. brevicompactum. The results clearly revealed that Polyunsaturated fatty acids (PUFAs) are long-chain fatty − the maximal lipid production of 8.014 ± 0.06 gL 1 acids containing two or more double bonds in their acyl (representing 57.6% lipid/dry biomass) was achieved by chains. Biosynthesis of PUFAs involves both methyl- the fungus when grown for 6 days at 30 °C under static directed and carboxyl-directed desaturases. The primary condition in a medium containing sunflower oil cake, product of fatty acid biosynthesis in oilseed crops is the NaNO3 and KCl at final concentrations of 8, 0.75 and 18-carbon monounsaturated oleic acid (C18:1–9). -

( Vaccinium Myrtillus L . ) And
Food Chemistry 354 (2021) 129517 Contents lists available at ScienceDirect Food Chemistry journal homepage: www.elsevier.com/locate/foodchem Analysis of composition, morphology, and biosynthesis of cuticular wax in wild type bilberry (Vaccinium myrtillus L.) and its glossy mutant Priyanka Trivedi a,1, Nga Nguyen a,1, Linards Klavins b, Jorens Kviesis b, Esa Heinonen c, Janne Remes c, Soile Jokipii-Lukkari a, Maris Klavins b, Katja Karppinen d, Laura Jaakola d,e, Hely Haggman¨ a,* a Department of Ecology and Genetics, University of Oulu, FI-90014 Oulu, Finland b Department of Environmental Science, University of Latvia, LV-1004 Riga, Latvia c Centre for Material Analysis, University of Oulu, FI-90014 Oulu, Finland d Department of Arctic and Marine Biology, UiT The Arctic University of Norway, NO-9037 Tromsø, Norway e NIBIO, Norwegian Institute of Bioeconomy Research, NO-1431 Ås, Norway ARTICLE INFO ABSTRACT Keywords: In this study, cuticular wax load, its chemical composition, and biosynthesis, was studied during development of Cuticular wax wild type (WT) bilberry fruit and its natural glossy type (GT) mutant. GT fruit cuticular wax load was comparable Fruit cuticle with WT fruits. In both, the proportion of triterpenoids decreased during fruit development concomitant with Gene expression increasing proportions of total aliphatic compounds. In GT fruit, a higher proportion of triterpenoids in cuticular Glossy type mutant wax was accompanied by a lower proportion of fatty acids and ketones compared to WT fruit as well as lower Triterpenoids Wax composition density of crystalloid structures on berry surfaces. Our results suggest that the glossy phenotype could be caused Chemical compounds studied in this article: by the absence of rod-like structures in GT fruit associated with reduction in proportions of ketones and fatty β-Amyrin (PubChem CID: 73145) acids in the cuticular wax. -

Component, Fatty Acid and Mineral Composition of Rice Bran Oil Extracted by Multistage with Hexane and Ethanol
INTERNATIONAL JOURNAL OF SCIENTIFIC & TECHNOLOGY RESEARCH VOLUME 6, ISSUE 11, NOVEMBER 2017 ISSN 2277-8616 Component, Fatty Acid And Mineral Composition Of Rice Bran Oil Extracted By Multistage With Hexane And Ethanol Fajriyati Mas’ud, Meta Mahendradatta, Amran Laga, Zainal Zainal Abstract: Rice bran oil (RBO) has been extracted from Celebes rice bran by multistage extraction with hexane solvent followed by ethanol to see the component, profile of fatty acids and mineral contained in both of them. As a comparison, RBO directly extracted with ethanol was also presented. Extraction process was performed using reflux method at 55oC, for 5 hours with bran and solvent ratio of 1:7. Analysis of components and fatty acids of RBO was conducted with GC-MS QP 2010 Shimadzu. Oleic, linoleic and palmitic were found dominant in first stage extraction by hexane with concentration of 3716.56, 1630.78 and 1021.89 mg/L, respectively. Palmitic (6.34 mg/L), lauric (4.78 mg/L), and linoleic (3.52 mg/L) were dominant in the second stage extraction by ethanol. Linoleic (28.85 mg/L), stearic (2.88 mg/L) and myristic (2.02 mg/L) were found in extracted directly by ethanol. RBO extracted with hexane had 18.6% of saturated fatty acid and 81.4% of unsaturated fatty acids, with ratio of saturated fatty acids : monounsaturated fatty acids: polyunsaturated fatty acids of approximately 1: 2.3 : 1.3. It contained about 56.7% of monounsaturated, 24.7% of polyunsaturated, and 18.6% of saturated fatty acids. In the present paper, we provide also an analysis of mineral composition of RBO by X-ray Spectrometer and melting point of RBO by Differential Scanning Calorimeter (DSC) instrument. -

Poster Session III, December 6, 2017
Neuropsychopharmacology (2017) 42, S476–S652 © 2017 American College of Neuropsychopharmacology. All rights reserved 0893-133X/17 www.neuropsychopharmacology.org Poster Session III a high-resolution research tomograph (HRRT), and struc- Palm Springs, California, December 3–7, 2017 tural (T1) MRI using a 3-Tesla scanner. PET data analyses were carried out using the validated 2-tissue compartment Sponsorship Statement: Publication of this supplement is model to determine the total volume of distribution (VT) of sponsored by the ACNP. [18F]FEPPA. Individual contributor disclosures may be found within the Results: Results show significant inverse associations (con- abstracts. Part 1: All Financial Involvement with a pharma- trolling for rs6971 genotype) between neuroinflammation ceutical or biotechnology company, a company providing (VTs) and cortical thickness in the right medial prefrontal clinical assessment, scientific, or medical products or compa- cortex [r = -.562, p = .029] and the left dorsolateral nies doing business with or proposing to do business with prefrontal cortex [r = -.629, p = .012](p values are not ACNP over past 2 years (Calendar Years 2014–Present); Part 2: corrected for multiple comparisons). No significant associa- Income Sources & Equity of $10,000 per year or greater tions were found with surface area. (Calendar Years 2014 - Present): List those financial relation- Conclusions: These results, while preliminary, suggest links ships which are listed in part one and have a value greater than between the microglial activation/neuroinflammation and $10,000 per year, OR financial holdings that are listed in part cortical thickness in the dorsolateral prefrontal cortex and one and have a value of $10,000 or greater as of the date of medial prefrontal cortex in AD patients. -
Rapastinel Flop Leaves Allergan in a Hole
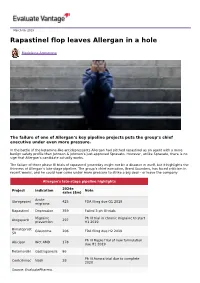
March 06, 2019 Rapastinel flop leaves Allergan in a hole Madeleine Armstrong The failure of one of Allergan’s key pipeline projects puts the group’s chief executive under even more pressure. In the battle of the ketamine-like antidepressants Allergan had pitched rapastinel as an agent with a more benign safety profile than Johnson & Johnson’s just-approved Spravato. However, unlike Spravato, there is no sign that Allergan’s candidate actually works. The failure of three phase III trials of rapastinel yesterday might not be a disaster in itself, but it highlights the thinness of Allergan’s late-stage pipeline. The group’s chief executive, Brent Saunders, has faced criticism in recent weeks, and he could now come under more pressure to strike a big deal – or leave the company. Allergan's late-stage pipeline highlights 2024e Project Indication Note sales ($m) Acute Ubrogepant 425 FDA filing due Q1 2019 migraine Rapastinel Depression 359 Failed 3 ph III trials Migraine Ph III trial in chronic migraine to start Atogepant 297 prevention H1 2019 Bimatoprost Glaucoma 206 FDA filing due H2 2019 SR Ph III Maple trial of new formulation Abicipar Wet AMD 178 due H1 2019 Relamorelin Gastroparesis 86 Ph III Aurora trial due to complete Cenicriviroc Nash 28 2020 Source: EvaluatePharma. Activist shareholders have long been agitating for change at Allergan, and the latest failure will only provide more ammunition to those who want to split the roles of chairman and chief exec amid dissatisfaction with the group's acquisition strategy, and with its stock hovering around a five-year low. -

WO 2017/066590 Al 20 April 2017 (20.04.2017) P O P CT
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2017/066590 Al 20 April 2017 (20.04.2017) P O P CT (51) International Patent Classification: (81) Designated States (unless otherwise indicated, for every A61K 38/06 (2006.01) A61K 31/519 (2006.01) kind of national protection available): AE, AG, AL, AM, A61 38/07 (2006.0V) A61K 31/551 (2006.01) AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, A61K 31/13 (2006.01) A61K 31/5513 (2006.01) BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DJ, DK, DM, A61K 31/135 (2006.01) A61P 25/24 (2006.01) DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, A61K 31/496 (2006.01) A61P 25/18 (2006.01) HN, HR, HU, ID, IL, IN, IR, IS, JP, KE, KG, KN, KP, KR, KW, KZ, LA, LC, LK, LR, LS, LU, LY, MA, MD, ME, (21) International Application Number: MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, PCT/US20 16/057071 OM, PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SA, (22) International Filing Date: SC, SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, 14 October 2016 (14.10.201 6) TN, TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, zw. (25) Filing Language: English (84) Designated States (unless otherwise indicated, for every (26) Publication Language: English kind of regional protection available): ARIPO (BW, GH, (30) Priority Data: GM, KE, LR, LS, MW, MZ, NA, RW, SD, SL, ST, SZ, 62/242,633 16 October 2015 (16. -

Biochemistry Prologue to Lipids
Paper : 05 Metabolism of Lipids Module: 01 Prologue to Lipids Principal Investigator Dr. Sunil Kumar Khare, Professor, Department of Chemistry, IIT-Delhi Paper Coordinator and Dr. Suaib Luqman, Scientist (CSIR-CIMAP) Content Writer & Assistant Professor (AcSIR) CSIRDr. Vijaya-CIMAP, Khader Lucknow Dr. MC Varadaraj Content Reviewer Prof. Prashant Mishra, Professor, Department of Biochemical Engineering and Biotechnology, IIT-Delhi 1 METABOLISM OF LIPIDS Biochemistry Prologue to Lipids DESCRIPTION OF MODULE Subject Name Biochemistry Paper Name 05 Metabolism of Lipids Module Name/Title 01 Prologue to Lipids 2 METABOLISM OF LIPIDS Biochemistry Prologue to Lipids 1. Objectives To understand what is lipid Why they are important How they occur in nature 2. Concept Map LIPIDS Fatty Acids Glycerol 3. Description 3.1 Prologue to Lipids In 1943, the term lipid was first used by BLOOR, a German biochemist. Lipids are heterogeneous group of compounds present in plants and animal tissues related either actually or potentially to the fatty acids. They are amphipathic molecules, hydrophobic in nature originated utterly or in part by thioesters (carbanion-based condensations of fatty acids and/or polyketides etc) or by isoprene units (carbocation-based condensations of prenols, sterols, etc). Lipids have the universal property of being: i. Quite insoluble in water (polar solvent) ii. Soluble in benzene, chloroform, ether (non-polar solvent) 3 METABOLISM OF LIPIDS Biochemistry Prologue to Lipids Thus, lipids include oils, fats, waxes, steroids, vitamins (A, D, E and K) and related compounds, such as phospholipids, triglycerides, diglycerides, monoglycerides and others, which are allied more by their physical properties than by their chemical assests.