Transfusion Guidelines
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Guidelines for Transfusions
Guidelines for Transfu- sion Prepared by: Community Transfusion Committee Lincoln, Nebraska CHAIR: Aina Silenieks, MD MEMBERS: L. Bausch, M.D. R. Burton, M.D. S. Dunder, M.D. D. Voigt, M.D. B. J. Wilson, M.D. COMMUNITY Becky Croner REPRESENTATIVES: Ellen DiSalvo Christa Engel Phyllis Ericson Kelly Gillaspie Pat Gilles Vic Grdina Jessica Henrichs Kelly Jensen Laurel McReynolds Christina Nickel Angela Novotny Kelley Thiemann Janet Wachter Jodi Wikoff Guidelines For Transfusion Community Transfusion Committee INTRODUCTION The Community Transfusion Committee is a multidisciplinary group that meets to monitor blood utilization practices, establish guidelines for transfusion and discuss relevant transfusion related topics. It is comprised of physicians from local hospitals, invited guests, and community representatives from the hospitals’ transfusion services, nursing services, perfusion services, health information management, and the Nebraska Community Blood Bank. These Guidelines for Transfusion are reviewed and revised biannually by the Community Trans- fusion Committee to ensure that the industry’s most current practices are promoted. The Guidelines are the standard by which utilization practices are evaluated. They are also de- signed to provide helpful information to assist physicians to provide appropriate blood compo- nent therapy to patients. Appendices have been added for informational purposes and are not to be used as guidance for clinical decision making. ADULT RED CELLS A. Indications 1. One of the following a. Hypovolemia and hypoxia (signs/symptoms: syncope, dyspnea, postural hypoten- sion, tachycardia, angina, or TIA) secondary to surgery, trauma, GI tract bleeding, or intravascular hemolysis, OR b. Evidence of acute loss of 15% of total blood volume or >750 mL blood loss, OR c. -

Platelet Transfusion
Lab Dept: Transfusion Services Test Name: PLATELET TRANSFUSION General Information Lab Order Codes: TPLT Special charges: HLA Matched-MHLA, AHLA PLA1 Negative – PLAT, APLA1 Platelet Crossmatching PLTX – CPLT Synonyms: Leukocyte Reduced Platelets; Random Platelets; Platelet Rich Plasma; Platelet concentrate; Leukocyte Reduced Platelet Pheresis; Single- Donor Platelets; SDP Platelet pheresis; Apheresis Platelets; LRPH CPT Codes: P9035 – Platelet pheresis, Leukocyte reduced 86806 – Lymphocytotoxicity assay, visual crossmatch, without titration 86813 – HLA Matched 86945 – Irradiation 86985 – Volume Reduction 86965 – Pooling 86903 – Platelet Antigen typing 86999 – Washing 86022 – Platelet Crossmatch Test Includes: Leukocyte Reduced Platelet Pheresis consists of platelets suspended in 200-300 mL of plasma collected by cytapheresis. Each unit contains at least 3 x 1011 platelets, and ≤5.0 x 106 leukocytes. One pheresis unit equals 5-6 random unit platelet concentrate. Logistics Test Indications: Refer to Guidelines for the Transfusion of Blood Components. Platelet Crossmatching or HLA-Matched Platelets may be useful for patients receiving repeated platelet transfusions who have become refractile, and for patients who repeatedly develop febrile non-hemolytic transfusion reactions after platelet transfusions. Volume Reduced Platelets are indicated in the event that ABO - incompatible platelets must be transfused due to availability or in patients with severe fluid restrictions. Refer to Blood Component Compatibility Chart Lab Testing Sections: Transfusion Services Phone Numbers: MIN Lab: 612-813-6824 STP Lab: 651-220-6558 Test Availability: Daily, 24 hours Turnaround Time: 1 - 2 hours Standard Dose/Volume: <10 kg: 10 – 15 mL/kg up to one unit or 50 mL maximum 10 – 15 kg: 1/3 pheresis unit 15 – 25 kg: 1/2 pheresis unit >25 kg: 1 pheresis unit Rate of Infusion: 10 minutes/unit, <4 hours Administration: Must be administered through a blood component administration filter. -

Blood Product Modifications: Leukofiltration, Irradiation and Washing
Blood Product Modifications: Leukofiltration, Irradiation and Washing 1. Leukocyte Reduction Definitions and Standards: o Process also known as leukoreduction, or leukofiltration o Applicable AABB Standards, 25th ed. Leukocyte-reduced RBCs At least 85% of original RBCs < 5 x 106 WBCs in 95% of units tested . Leukocyte-reduced Platelet Concentrates: At least 5.5 x 1010 platelets in 75% of units tested < 8.3 x 105 WBCs in 95% of units tested pH≥6.2 in at least 90% of units tested . Leukocyte-reduced Apheresis Platelets: At least 3.0 x 1011 platelets in 90% of units tested < 5.0 x 106 WBCs 95% of units tested pH≥6.2 in at least 90% of units tested Methods o Filter: “Fourth-generation” filters remove 99.99% WBCs o Apheresis methods: most apheresis machines have built-in leukoreduction mechanisms o Less efficient methods of reducing WBC content . Washing, deglycerolizing after thawing a frozen unit, centrifugation . These methods do not meet requirement of < 5.0 x 106 WBCs per unit of RBCs/apheresis platelets. Types of leukofiltration/leukoreduction o “Pre-storage” . Done within 24 hours of collection . May use inline filters at time of collection (apheresis) or post collection o “Pre-transfusion” leukoreduction/bedside leukoreduction . Done prior to transfusion . “Bedside” leukoreduction uses gravity-based filters at time of transfusion. Least desirable given variability in practice and absence of proficiency . Alternatively performed by transfusion service prior to issuing Benefits of leukoreduction o Prevention of alloimmunization to donor HLA antigens . Anti-HLA can mediate graft rejection and immune mediated destruction of platelets o Leukoreduced products are indicated for transplant recipients or patients who are likely platelet transfusion dependent o Prevention of febrile non-hemolytic transfusion reactions (FNHTR) . -

Important Information for Female Platelet Donors
Important Information for Female Platelet Donors We are grateful for the support you provide our community blood program and especially appreciate your willingness to help save lives as a volunteer platelet donor. We also take our responsibility to provide a safe and adequate blood supply very seriously and need to share the following information regarding a change to our donor eligibility criteria for female platelet donors. We recently began performing a Human Leukocyte Antigen (HLA) antibody test on each of our current female platelet donors who have ever been pregnant. In addition, we modified our Medical History Questionnaire to ask donors whether they have been pregnant since their last donation. Platelet donors who respond yes to that question will be screened for HLA antibodies. Platelet donors will also be retested after every subsequent pregnancy. These adjustments are being made as part of our effort to reduce occurrences of Transfusion-Related Acute Lung Injury (TRALI). TRALI is a rare but serious complication of blood transfusions most commonly thought to be caused by a reaction to HLA antibodies present in the donor’s plasma. When transfused, these antibodies can sometimes cause plasma to leak into the patient’s lungs, creating fluid accumulation — a condition referred to as acute pulmonary edema. Female donors who have been pregnant are more likely than others to have these HLA antibodies in their plasma. Once the antibodies develop, they are present forever. The antibodies could be harmful if transfused into certain patients. The antibodies are present in plasma — and platelet donations contain a high volume of plasma, so our current efforts are directed at screening blood samples from female platelet donors to test for the HLA antibody. -

Transfusion Triggers for Platelets and Other Blood Products Sunil Karanth Indian Journal of Critical Care Medicine (2019): 10.5005/Jp-Journals-10071-23250
INVITED ARTICLE Transfusion Triggers for Platelets and Other Blood Products Sunil Karanth Indian Journal of Critical Care Medicine (2019): 10.5005/jp-journals-10071-23250 INTRODUCTION Department of Intensive Care Unit, Manipal Hospital, Bengaluru, Transfusion of whole blood is not associated with any significant Karnataka, India benefit, rather can be harmful. Significant advances have been Corresponding Author: Sunil Karanth, Department of Intensive made in transfusion medicine, facilitating the use of blood product Care Unit, Manipal Hospital, Bengaluru, Karnataka, India, e-mail: or component therapy than use of whole blood (Fig. 1). In 2009, a [email protected] report on Serious Hazards of Transfusion in the UK, estimated that How to cite this article: Karanth S. Transfusion Triggers for Platelets a total of 3 million units of blood components were released. The and Other Blood Products. Indian J Crit Care Med 2019;23(Suppl requirement of the same in South-East Asia is much higher to the 3):S189–S190. tune of 15 million units annually. The current recommendation Source of support: Nil is to use blood only in life-threatening situations, rather than to Conflict of interest: None normalize abnormal numbers. PLATELETS Platelets are derived from the buffy coat of whole blood donations. A pooled platelet concentrate includes pooled buffy-coat derived platelets from four whole-blood donations suspended in platelet additive solution and plasma of one of the four donors. It contains 240000 platelets pooled from 4–6 donors. A Single donor platelet is derived from a single-donor by a process of apheresis. In view of the lesser number of donors and the theoretical advantage of involving a single-donor platelet (SDP) may be preferred over the use of platelets from multiple donors. -

Blood-Platelet-Orders-Outpatient-V3
Blood / Platelet Orders Outpt v3 USE THIS ORDER SET FOR OUTPATIENT NON URGENT BLOOD OR PLATELET TRANSFUSION 24 Hours advanced notice required for infusion center NO MORE THAN 2 UNITS OF RED BLOOD CELLS CAN BE INFUSED IN THE INFUSION CENTER PER DAY BMH Outpatient Infusion Center: Fax completed order set to 843-522-7313/Phone 843-522-7680 Hospital Outpatient: Fax Completed order set to Registration at 843-522-5741 and notify Nursing Supervisor at 843-522-7653 Service Designation THIS IS NOT AN ADMISSION SET Patient Name ___________________________________ Patient DOB ____________ Service Designation Blood Platelet Outpatient Attending ___________________________________ Date Requested ___________________________________ Status Outpatient Check Appropriate Diagnosis Below: [ Anemia of chronic renal disease Anemia related to chemotherapy Aplastic anemia Anemia related to cancer Anemia related to blood loss Sickle cell disease Anemia unspecified Thombocytopenia (platelets) Other _________________________ ] Allergies Update Allergies in the Summary Panel in MEDITECH Special Requirement: (REQUIRES SPECIAL ORDER ONE DAY IN ADVANCE) Special Requirement RBC or Platelets REQUIRES SPECIAL ORDER ONE DAY IN ADVANCE [ Irradiated CMV Negative HgBS Negative ] Medications diphenhydrAMINE HCl (Benadryl) 25 mg orally single dose premedicate prior to transfusion diphenhydrAMINE HCl (Benadryl) 50 mg orally single dose premedicate prior to transfusion diphenhydrAMINE HCl (Benadryl) 25 mg intravenously single dose premedicate prior to transfusion diphenhydrAMINE -

CD59: a Long-Known Complement Inhibitor Has Advanced to a Blood Group System
B LOOD G ROUP R EVIEW CD59: A long-known complement inhibitor has advanced to a blood group system C. Weinstock, M. Anliker, and I. von Zabern The blood group system number 35 is based on CD59, a 20-kDa by Zalman et al.1 from the group of H.J. Muller-Eberhard in La membrane glycoprotein present on a large number of different Jolla, California, and in 1988 by Schönermark et al. from the cells, including erythrocytes. The major function of CD59 is to group of G.M. Hänsch in Heidelberg (Germany).2 This inhibitor protect cells from complement attack. CD59 binds to complement components C8 and C9 and prevents the polymerization of C9, was published under the designations “HRF (homologous which is required for the formation of the membrane attack restriction factor)” and “C8bp (C8 binding protein),” complex (MAC). Other functions of CD59 in cellular immunity respectively, to describe its properties.1,2 “C8bp” indicates the are less well defined. CD59 is inserted into the membrane by a glycosylphosphatidylinositol (GPI) anchor. A defect of this anchor binding capacity for C8. The name “HRF” points to a species causes lack of this protein from the cell membrane, which leads to incompatibility of this “factor” that provides protection from an enhanced sensitivity towards complement attack. Patients with complement attack more effectively in a homologous (e.g., paroxysmal nocturnal hemoglobinuria (PNH) harbor a varying human erythrocytes as a target of human complement) than in percentage of red blood cell clones with a defect in GPI-anchored proteins, including CD59. The most characteristic symptoms of a heterologous system (e.g., human erythrocytes as a target of this disease are episodes of hemolysis and thromboses. -
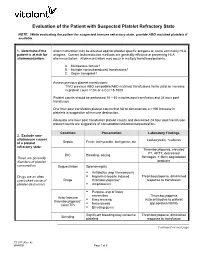
Platelet Transfusion Refractoriness
Evaluation of the Patient with Suspected Platelet Refractory State NOTE: While evaluating the patient for suspected immune refractory state, provide ABO matched platelets if available. 1. Determine if the Alloimmunization may be directed against platelet specific antigens or, more commonly HLA patient is at risk for antigens. Current leukoreduction methods are generally effective at preventing HLA alloimmunization: alloimmunization. Alloimmunization may occur in multiply transfused patients. A. Multiparous female? B. Multiple nonleukoreduced transfusions? C. Organ transplant? Assess previous platelet transfusions: TWO previous ABO compatible/ABO matched transfusions fail to yield an increase in platelet count >10K or a CCI >5-7500 Platelet counts should be performed 10 – 60 minutes post transfusion and 24 hour post transfusion. One hour post transfusion platelet counts that fail to demonstrate a >10K increase in platelets is suggestive of immune destruction. Adequate one hour post transfusion platelet counts and decreased 24 hour post transfusion platelet counts are suggestive of consumption/utilization/sequestration. Condition Presentation Laboratory Findings 2. Exclude non- alloimmune causes Leukocytosis, +cultures Sepsis Fever, tachycardia, tachypnea, etc. of a platelet refractory state: Thrombocytopenia, elevated PT, APTT, decreased DIC Bleeding, oozing These are generally fibrinogen, + fibrin degradation disorders of platelet products consumption. Sequestration Splenomegaly . Antibiotics (esp Vancomycin) Drugs are an often . Heparin (Heparin induced Thrombocytopenia, diminished overlooked cause of Drugs thrombocytopenia)* response to transfusion platelet destruction. Amphotericin . Purpura, esp of lower extremities Thrombocytopenia Auto-Immune . Easy bruising Auto antibodies to platelet thrombocytopenia* Nose bleeds glycoprotein IIb/IIIa (aka ITP) . Bleeding gums Significant bleeding may consume Thrombocytopenia, diminished Bleeding platelets response to transfusion Continued on next page TS 017 (Rev. -

Comparing Platelet Compatibility to Red Cell Compatibility Protocols
Review: comparing platelet compatibility to red cell compatibility protocols S. ROLIH Between 1971 and 1980, the number of platelet con- Incidence of Alloimmunization From centrates transfused in the United States increased from Transfusion 0.4 to 2.8 million units.1 That number increased to near- Each RBC or platelet transfusion has the potential to ly 5 million in 1987 and continues to increase in the influence the outcome of future transfusions of the 1990s. The increase in platelet transfusions has led to a same products by inducing the formation of alloanti- concomitant rise in the incidence of platelet alloimmu- bodies. For RBC transfusions, in which ABO and D nization. As a consequence, many transfusion services group-compatible units are routinely given, this is not a or blood product providers have developed platelet significant problem. Only 0.3 percent to 1.3 percent of compatibility protocols to select platelet components all RBC transfusion recipients produce alloantibodies, that will have acceptable survival when transfused to although in selected recipient populations, such as alloimmunized patients. Most serologists are familiar patients with thalassemia or sickle cell anemia, the with compatibility tests used to select red blood cells alloimmunization rate may be as high as 30 percent (RBCs) for transfusion. These tests are performed before because of chronic transfusions.2 Overall, decreased sur- transfusion, whether or not alloimmunization has vival of transfused RBCs due to alloimmunization occurs occurred. Platelet compatibility tests, in contrast, are infrequently. Once alloimmunization occurs, premature implemented only after alloimmunization and develop- loss of future transfused RBCs is prevented by the selec- ment of the refractory state. -

Outpatient Blood Platelet Orders
Blood / Platelet Orders Outpt v5 USE THIS ORDER SET FOR OUTPATIENT NON URGENT BLOOD OR PLATELET TRANSFUSION 24 Hours advanced notice required for infusion center NO MORE THAN 2 UNITS OF RED BLOOD CELLS CAN BE INFUSED AS AN OUTPATIENT PER DAY BMH Outpatient Infusion Center: Fax completed order set to 843-522-7313/Phone 843-522-7680 Hospital Outpatient: Fax Completed order set to Registration at 843-522-5741 and notify Nursing Supervisor at 843-522-7653 ALL INFORMATION BELOW IS REQUIRED BY ORDERING PHYSICIAN Service Designation THIS IS NOT AN ADMISSION SET Patient Name ___________________________________ Patient DOB ____________ Service Designation Blood Platelet Outpatient Attending / Ordering Physician ______________________________ Date Requested ___________________________________ Status Outpatient Check Appropriate Diagnosis Below: [ Anemia of chronic renal disease Anemia related to chemotherapy Myelodysplasia Anemia related to cancer Anemia related to blood loss Sickle cell disease Anemia unspecified Thombocytopenia (platelets) Other _________________________ ] Allergies Update Allergies in the Summary Panel in MEDITECH Special Requirement: (REQUIRES SPECIAL ORDER ONE DAY IN ADVANCE) Special Requirement RBC or Platelets REQUIRES SPECIAL ORDER ONE DAY IN ADVANCE [ Irradiated CMV Negative HgBS Negative ] Vital Signs Per BMH Policy, Blood and Blood Component Administration, 07.02 Diet Mediterranean Style Diet 2 Gm Sodium Diet Regular 7 Diet 1800 kcal Consistent Carbohydrate Diet Other Dietper patient choice ____________________ Medications -

Essential Thrombocythemia Facts No
Essential Thrombocythemia Facts No. 12 in a series providing the latest information for patients, caregivers and healthcare professionals www.LLS.org • Information Specialist: 800.955.4572 Introduction Highlights Essential thrombocythemia (ET) is one of several l Essential thrombocythemia (ET) is one of a related “myeloproliferative neoplasms” (MPNs), a group of closely group of blood cancers known as “myeloproliferative related blood cancers that share several features, notably the neoplasms” (MPNs) in which cells in the bone “clonal” overproduction of one or more blood cell lines. marrow that produce the blood cells develop and All clonal disorders begin with one or more changes function abnormally. (mutations) to the DNA in a single cell; the altered cells in l ET begins with one or more acquired changes the marrow and the blood are the offspring of that one (mutations) to the DNA of a single blood-forming mutant cell. Other MPNs include polycythemia vera and cell. This results in the overproduction of blood cells, myelofibrosis. especially platelets, in the bone marrow. The effects of ET result from uncontrolled blood cell l About half of individuals with ET have a mutation production, notably of platelets. Because the disease arises of the JAK2 (Janus kinase 2) gene. The role that this from a change to an early blood-forming cell that has the mutation plays in the development of the disease, capacity to form red cells, white cells and platelets, any and the potential implications for new treatments, combination of these three cell lines may be affected – and are being investigated. usually each cell line is affected to some degree. -

Platelet Transfusion in the Real Life
4 Editorial Commentary Page 1 of 4 Platelet transfusion in the real life Pilar Solves1,2 1Blood Bank, Hematology Service, Hospital Universitari I Politècnic la Fe, Valencia, Spain; 2CIBERONC, Instituto Carlos III, Madrid, Spain Correspondence to: Pilar Solves. Blood Bank, Hematology Unit, Hospital Universitari I Politècnic La Fe, Avda Abril Martorell, 106, Valencia 46026, Spain. Email: [email protected]. Provenance and Peer Review: This article was commissioned by the editorial office,Annals of Blood. The article did not undergo external peer review. Comment on: Gottschall J, Wu Y, Triulzi D, et al. The epidemiology of platelet transfusions: an analysis of platelet use at 12 US hospitals. Transfusion 2020;60:46-53. Received: 06 April 2020. Accepted: 24 April 2020; Published: 30 June 2020. doi: 10.21037/aob-20-30 View this article at: http://dx.doi.org/10.21037/aob-20-30 Platelet transfusion is a common practice in thrombocytopenic period, between January 2013 and December 2016. patients for preventing or treating hemorrhages. Platelets The results provide a general view on current platelet are mostly transfused to patients diagnosed of onco- transfusion practice in US. A total of 163,719 platelets hematological diseases and/or undergoing hematopoietic representing between 3% and 5% of all platelets stem cell transplantation (1). With the aim to help transfused in the country, were transfused into 31821 physicians to take the most accurate decisions on platelet patients of whom 60.5% were males. More than 60% of transfusion, some guidelines have been developed (2-6). platelets were from single donor apheresis and 72.5% The scientific evidence supporting the recommendations were irradiated.