Host Fishes and Life History of the Round Hickorynut (Obovaria Subrotunda)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Caracara Cheriway
Rare Animal Fact Sheet IMBIV31060 Louisiana Department of Wildlife and Fisheries Natural Heritage Program Obovaria unicolor Alabama Hickorynut Identification: This mussel has a round or elliptical shape; shell is nearly smooth, shiny, brown to yellowish brown with rays, young shells are green with green rays; mother-of-pear is pink but sometimes white or blue. Measurements: A small mussel, 1.5 to 2 inches in length. Taxonomic comments: No recognized subspecies. Status: Global ranking is G3 and state rank is S1. Habitat: Sand and gravel bottoms in river systems. Range: Eastern gulf drainages throughout Alabama, Louisiana, Mississippi, and Oklahoma. Food habits*: Mussels are continually pumping water through their siphon. Their diet is composed of the various micropscopic plants and animals from the water and organic matter from stream bottoms that they filter from this water. Reproduction: Sexes are separate. No information on host fish available. Reason for decline: 1) The damming of stream and rivers results in the loss of mussel and host fish habitat. 2) Channelization of large stream habitat. 3) Declining water quality as a result of siltation (reduces oxygen level) and heavy recreational use of habitat. Interesting facts: This species is common only in the Sipsey River in Alabama. It is believed to have been uncommon or historically rare throughout the rest of its range. * Indicates generalized information for freshwater mussels in the absence of information specific to this species Rare Animal Fact Sheet IMBIV31060 Known distribution in Louisiana: Dates of documented observations are: 1977, 1979, 1988, and 1994 References McCullagh, W.H., J.D. Williams, S.W. -

The Gut Microbiome of Freshwater Unionidae Mussels Is Determined by Host Species and Is Selectively Retained from Filtered Seston
University of Mississippi eGrove Faculty and Student Publications Biology 11-1-2019 The gut microbiome of freshwater Unionidae mussels is determined by host species and is selectively retained from filtered seston Eric A. Weingarten University of Mississippi Carla L. Atkinson University of Alabama Colin R. Jackson University of Mississippi Follow this and additional works at: https://egrove.olemiss.edu/biology_facpubs Recommended Citation Weingarten, E. A., Atkinson, C. L., & Jackson, C. R. (2019). The gut microbiome of freshwater Unionidae mussels is determined by host species and is selectively retained from filtered seston. PLOS ONE, 14(11), e0224796. https://doi.org/10.1371/journal.pone.0224796 This Article is brought to you for free and open access by the Biology at eGrove. It has been accepted for inclusion in Faculty and Student Publications by an authorized administrator of eGrove. For more information, please contact [email protected]. RESEARCH ARTICLE The gut microbiome of freshwater Unionidae mussels is determined by host species and is selectively retained from filtered seston 1 2 1 Eric A. Weingarten , Carla L. Atkinson , Colin R. JacksonID * 1 Department of Biology, University of Mississippi, University, Mississippi, United States of America, 2 Department of Biological Sciences, University of Alabama, Tuscaloosa, Alabama, United States of America * [email protected] a1111111111 a1111111111 a1111111111 Abstract a1111111111 a1111111111 Freshwater mussels are a species-rich group of aquatic invertebrates that are among the most endangered groups of fauna worldwide. As filter-feeders that are constantly exposed to new microbial inoculants, mussels represent an ideal system to investigate the effects of species or the environment on gut microbiome composition. -

Indiana Species April 2007
Freshwater Mussels of Indiana April 2007 The Wildlife Diversity Section (WDS) is responsible for the conservation and management of over 750 species of nongame and endangered wildlife. The list of Indiana's species was compiled by WDS biologists based on accepted taxonomic standards. The list will be periodically reviewed and updated. References used for scientific names are included at the bottom of this list. ORDER FAMILY GENUS SPECIES COMMON NAME STATUS* Unionoida Unionidae Actinonaias ligamentina mucket Alasmidonta marginata elktoe Alasmidonta viridis slippershell mussel Amblema plicata threeridge Anodonta suborbiculata flat floater Anodontoides ferussacianus cylindrical papershell Arcidens confragosus rock pocketbook Cyclonaias tuberculata purple wartyback Cyprogenia stegaria fanshell SE/FE Ellipsaria lineolata butterfly Elliptio crassidens elephantear Elliptio dilatata spike Epioblasma obliquata perobliqua white catspaw SE/FE Epioblasma torulosa rangiana northern riffleshell SE/FE Epioblasma torulosa torulosa tubercled blossom SE/FE Epioblasma triquetra snuffbox SE Fusconaia ebena ebonyshell Fusconaia flava Wabash pigtoe Fusconaia subrotunda longsolid SE Lampsilis abrupta pink mucket SE/FE Lampsilis cardium plain pocketbook Lampsilis fasciola wavyrayed lampmussel SC Lampsilis ovata pocketbook Lampsilis siliquoidea fatmucket Lampsilis teres yellow sandshell Lasmigona complanata white heelsplitter Lasmigona compressa creek heelsplitter Lasmigona costata flutedshell Leptodea fragilis fragile papershell (Freshwater Mussels of Indiana -

A Thesis Entitled Molecular, Morphological, and Biogeographic Resolution of Cryptic Taxa in the Greenside Darter Etheostoma Blen
A Thesis Entitled Molecular, morphological, and biogeographic resolution of cryptic taxa in the Greenside Darter Etheostoma blennioides complex By Amanda E. Haponski Submitted as partial fulfillment of the requirements for The Master of Science Degree in Biology (Ecology-track) ____________________________ Advisor: Dr. Carol A. Stepien ____________________________ Committee Member: Dr. Timothy G. Fisher ____________________________ Committee Member: Dr. Johan F. Gottgens ____________________________ College of Graduate Studies The University of Toledo December 2007 Copyright © 2007 This document is copyrighted material. Under copyright law, no parts of this document may be reproduced without the expressed permission of the author. An Abstract of Molecular, morphological, and biogeographic resolution of cryptic taxa in the Greenside Darter Etheostoma blennioides complex Amanda E. Haponski Submitted as partial fulfillment of the requirements for The Master of Science Degree in Biology (Ecology-track) The University of Toledo December 2007 DNA sequencing has led to the resolution of many cryptic taxa, which are especially prevalent in the North American darter fishes (Family Percidae). The Greenside Darter Etheostoma blennioides commonly occurs in the lower Great Lakes region, where two putative subspecies, the eastern “Allegheny” type E. b. blennioides and the western “Prairie” type E. b. pholidotum , overlap. The objective of this study was to test the systematic identity and genetic divergence distinguishing the two subspecies in areas of sympatry and allopatry in comparison to other subspecies and close relatives. DNA sequences from the mtDNA cytochrome b gene and control region and the nuclear S7 intron 1 comprising a total of 1,497 bp were compared from 294 individuals across 18 locations, including the Lake Erie basin and the Allegheny, Meramec, Obey, Ohio, Rockcastle, Susquehanna, and Wabash River systems. -

Iowa Darter Etheostoma Exile ILLINOIS RANGE
Iowa darter Etheostoma exile Kingdom: Animalia FEATURES Phylum: Chordata The Iowa darter averages about two and three- Class: Osteichthyes fourths inches in length. It is a brown or green- Order: Perciformes brown fish with eight to 10 dark marks on the back and 10 to 14 dark blotches on the side separated by Family: Percidae red spaces. There is a dark, teardrop mark under the ILLINOIS STATUS eye and a dark bar in front of the eye, as well as bars on the fins. The lateral line is short, extending to common, native about the second dorsal fin. There are two spines in the anal fin. The cheeks have scales. The breeding male has a blue tint to the back, green side blotches separated by rust-red spaces, wide bands of blue and orange in the first dorsal fin and orange along the lower sides. BEHAVIORS The Iowa darter may be found in glacial lakes in northeastern Illinois, a few streams in northern Illinois and a few limestone quarries in Vermilion County. It lives in clear lakes, sloughs and creeks that have many aquatic plants. In streams it can be found in quiet pools over a mud or clay bottom with dead material and brush. Spawning occurs in April in shallow water over roots, vegetation or debris. The young Iowa darter eats plankton, while the adult feeds on immature insects and small crustaceans. ILLINOIS RANGE © Illinois Department of Natural Resources. 2020. Biodiversity of Illinois. Unless otherwise noted, photos and images © Illinois Department of Natural Resources. female © Konrad P. Schmidt, University of Minnesota male © Konrad P. -
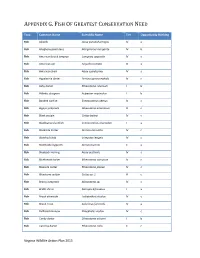
Fish of Greatest Conservation Need
APPENDIX G. FISH OF GREATEST CONSERVATION NEED Taxa Common Name Scientific Name Tier Opportunity Ranking Fish Alewife Alosa pseudoharengus IV a Fish Allegheny pearl dace Margariscus margarita IV b Fish American brook lamprey Lampetra appendix IV c Fish American eel Anguilla rostrata III a Fish American shad Alosa sapidissima IV a Fish Appalachia darter Percina gymnocephala IV c Fish Ashy darter Etheostoma cinereum I b Fish Atlantic sturgeon Acipenser oxyrinchus I b Fish Banded sunfish Enneacanthus obesus IV c Fish Bigeye jumprock Moxostoma ariommum III c Fish Black sculpin Cottus baileyi IV c Fish Blackbanded sunfish Enneacanthus chaetodon I a Fish Blackside darter Percina maculata IV c Fish Blotched chub Erimystax insignis IV c Fish Blotchside logperch Percina burtoni II a Fish Blueback Herring Alosa aestivalis IV a Fish Bluebreast darter Etheostoma camurum IV c Fish Blueside darter Etheostoma jessiae IV c Fish Bluestone sculpin Cottus sp. 1 III c Fish Brassy Jumprock Moxostoma sp. IV c Fish Bridle shiner Notropis bifrenatus I a Fish Brook silverside Labidesthes sicculus IV c Fish Brook Trout Salvelinus fontinalis IV a Fish Bullhead minnow Pimephales vigilax IV c Fish Candy darter Etheostoma osburni I b Fish Carolina darter Etheostoma collis II c Virginia Wildlife Action Plan 2015 APPENDIX G. FISH OF GREATEST CONSERVATION NEED Fish Carolina fantail darter Etheostoma brevispinum IV c Fish Channel darter Percina copelandi III c Fish Clinch dace Chrosomus sp. cf. saylori I a Fish Clinch sculpin Cottus sp. 4 III c Fish Dusky darter Percina sciera IV c Fish Duskytail darter Etheostoma percnurum I a Fish Emerald shiner Notropis atherinoides IV c Fish Fatlips minnow Phenacobius crassilabrum II c Fish Freshwater drum Aplodinotus grunniens III c Fish Golden Darter Etheostoma denoncourti II b Fish Greenfin darter Etheostoma chlorobranchium I b Fish Highback chub Hybopsis hypsinotus IV c Fish Highfin Shiner Notropis altipinnis IV c Fish Holston sculpin Cottus sp. -

Ambloplites Constellatus, a New Species of Rock Bass from the Ozark Upland of Arkansas and Missouri with a Review of Western Rock Bass Populations
Ambloplites constellatus, a New Species of Rock Bass from the Ozark Upland of Arkansas and Missouri with a Review of Western Rock Bass Populations ROBERT C. CASHNER and ROYAL D. SUTTKUS V Reprinted from THE AMERICAN MIDLAND NATURALIST Vol. 98, No. 1, July, 1977, pp. 147-161 University of Notre Dame Press Notre Dame, Indiana Ambloplites constellatus, a New Species of Rock Bass from the Ozark Upland of Arkansas and Missouri with a Review of Western Rock Bass Populations' ROBERT C. CASHNER Department of Biological Sciences, University of New Orleans, New Orleans, Louisiana 70122 and ROYAL D. SUTTKUS Tulane University, Museum of Natural History, Belle Chasse, Louisiana 70037 ABSTRACT: A new species of rock bass, Ambloplites constellatus, is described from the upland section of the White River in Arkansas and Missouri. It is compared with the closely related northern rock bass (A. rupestris) from Missouri and Meramec river populations, the southern rock bass (A. ariommus) from the Ouachita and Little river drainages, and with other western rock bass populations of undetermined status. Ambloplites constellatus is distinguished from its congeners by its freckled color pattern and slender body form. Ambloplites constellatus occurs throughout the upper White River. There are two records of the species from the Osage River drainage in Missouri. INTRODUCTION In his study of Missouri fishes, Pflieger (1971) noted that rock bass from the upper White River system differed strikingly in color pattern from other Missouri populations. Based on our examination of mate- rial from throughout the Ozark Upland province, as well as other western rock bass populations, we describe the upper White River population as a new species, Ambloplites constellatus, the Ozark rock bass. -

ECOLOGY of NORTH AMERICAN FRESHWATER FISHES
ECOLOGY of NORTH AMERICAN FRESHWATER FISHES Tables STEPHEN T. ROSS University of California Press Berkeley Los Angeles London © 2013 by The Regents of the University of California ISBN 978-0-520-24945-5 uucp-ross-book-color.indbcp-ross-book-color.indb 1 44/5/13/5/13 88:34:34 AAMM uucp-ross-book-color.indbcp-ross-book-color.indb 2 44/5/13/5/13 88:34:34 AAMM TABLE 1.1 Families Composing 95% of North American Freshwater Fish Species Ranked by the Number of Native Species Number Cumulative Family of species percent Cyprinidae 297 28 Percidae 186 45 Catostomidae 71 51 Poeciliidae 69 58 Ictaluridae 46 62 Goodeidae 45 66 Atherinopsidae 39 70 Salmonidae 38 74 Cyprinodontidae 35 77 Fundulidae 34 80 Centrarchidae 31 83 Cottidae 30 86 Petromyzontidae 21 88 Cichlidae 16 89 Clupeidae 10 90 Eleotridae 10 91 Acipenseridae 8 92 Osmeridae 6 92 Elassomatidae 6 93 Gobiidae 6 93 Amblyopsidae 6 94 Pimelodidae 6 94 Gasterosteidae 5 95 source: Compiled primarily from Mayden (1992), Nelson et al. (2004), and Miller and Norris (2005). uucp-ross-book-color.indbcp-ross-book-color.indb 3 44/5/13/5/13 88:34:34 AAMM TABLE 3.1 Biogeographic Relationships of Species from a Sample of Fishes from the Ouachita River, Arkansas, at the Confl uence with the Little Missouri River (Ross, pers. observ.) Origin/ Pre- Pleistocene Taxa distribution Source Highland Stoneroller, Campostoma spadiceum 2 Mayden 1987a; Blum et al. 2008; Cashner et al. 2010 Blacktail Shiner, Cyprinella venusta 3 Mayden 1987a Steelcolor Shiner, Cyprinella whipplei 1 Mayden 1987a Redfi n Shiner, Lythrurus umbratilis 4 Mayden 1987a Bigeye Shiner, Notropis boops 1 Wiley and Mayden 1985; Mayden 1987a Bullhead Minnow, Pimephales vigilax 4 Mayden 1987a Mountain Madtom, Noturus eleutherus 2a Mayden 1985, 1987a Creole Darter, Etheostoma collettei 2a Mayden 1985 Orangebelly Darter, Etheostoma radiosum 2a Page 1983; Mayden 1985, 1987a Speckled Darter, Etheostoma stigmaeum 3 Page 1983; Simon 1997 Redspot Darter, Etheostoma artesiae 3 Mayden 1985; Piller et al. -
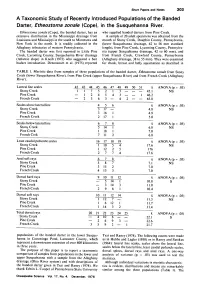
A Taxonomic Study of Recently Introduced Populations of The
Short Papers and Notes 303 A TaxonomicStudy of RecentlyIntroduced Populations of the Banded Darter,Etheostoma zonale (Cope), in the Susquehanna River. Etheostoma zonale (Cope), the banded darter, has an who supplied banded darters from Pine Creek. extensive distribution in the Mississippi drainage from A sample of 20 adult specimens was obtained from the Louisiana and Mississippi in the south to Minnesota and mouth of Stony Creek, Dauphin County, Pennsylvania, New York in the north. It is readily collected in the (lower Susquehanna drainage, 42 to 56 mm standard Allegheny tributaries of western Pennsylvania. length), from Pine Creek, Lycoming County, Pennsylva- The banded darter was first reported in Little Pine nia (upper Susquehanna drainage, 42 to 60 mm), and Creek, Lycoming County, Susquehanna River drainage from French Creek, Crawford County, Pennsylvania (Atlantic slope) in Kneib (1972) who suggested a bait (Allegheny drainage, 38 to 55 mm). They were examined bucket introduction. Denoncourt et al. (1975) reported for cheek, breast and belly squamation as described in TABLE 1. Meristic data from samples of three populations of the banded darter, Etheostoma zonale from Stony Creek (lower Susquehanna River), from Pine Creek (upper Susquehanna River) and from French Creek (Allegheny River). Lateral line scales 42 43 44 45 46 47 48 49 50 51 k ANOVA (p = .05) Stony Creek 1 1 7 5 2 1 3 - -- 45.1 NS Pine Creek - 1 2 4 5 4 3 - - 1 46.2 French Creek - 2 3 6 3 - 4 2 - - 45.8 Scales above lateral line 4 5 6 R ANOVA (p = .05) Stony Creek 3 -

AN ANALYSIS of SEXUAL DIMORPHISM in LENGTH and WEIGHT RELATIONSHIPS of IOWA DARTERS Margaret a Harings Northland College, CB: 853 1411 Ellis Ave Ashland, WI 54806
AN ANALYSIS OF SEXUAL DIMORPHISM IN LENGTH AND WEIGHT RELATIONSHIPS OF IOWA DARTERS Margaret A Harings Northland College, CB: 853 1411 Ellis Ave Ashland, WI 54806 ABSTRACT Iowa darters, Etheostoma exile, are commonly found throughout the Lake Superior watershed. However, given their abundance and non-game status, little is known about basic life history characteristics of these fish. Iowa darters were seined from Inch Lake (Bayfield County, WI) in May of 2010 and 2011 to assess sexual dimorphism in sizes. Collected fish were immediately frozen and later thawed and measured for total length (TL) and total weight (TW), and dissected to remove the gonads which were also weighed. Somatic weight (SW) was calculated for each fish. The mean TL of males and females did not differ. There was a weak difference in the relationships between log(TW) and log(TL) between male and female Iowa darters. There was a strong difference in the relationships between the log(GW) and log(TL) for males and females. However, a significant difference did not exist between log(somatic weight) and log(TL) between males and females. The weak difference in the TL-TW relationship between males and females appear to be due to the strong difference in the TL-GW relationship between the sexes. Additional research is planned to determine whether differences between males and females occur in age structure and diet. INTRODUCTION Iowa darters are small fish that are typically found in clear to slightly turbid, light brown water of small lakes, bogs, and small streams (Becker 1983). The range of Iowa darters extends east to New York, west to Montana, north to southern Canada, and south to portions of Illinois (Scott and Crossman 1973; Lee and Gilbert 1978). -

Summary Report of Freshwater Nonindigenous Aquatic Species in U.S
Summary Report of Freshwater Nonindigenous Aquatic Species in U.S. Fish and Wildlife Service Region 4—An Update April 2013 Prepared by: Pam L. Fuller, Amy J. Benson, and Matthew J. Cannister U.S. Geological Survey Southeast Ecological Science Center Gainesville, Florida Prepared for: U.S. Fish and Wildlife Service Southeast Region Atlanta, Georgia Cover Photos: Silver Carp, Hypophthalmichthys molitrix – Auburn University Giant Applesnail, Pomacea maculata – David Knott Straightedge Crayfish, Procambarus hayi – U.S. Forest Service i Table of Contents Table of Contents ...................................................................................................................................... ii List of Figures ............................................................................................................................................ v List of Tables ............................................................................................................................................ vi INTRODUCTION ............................................................................................................................................. 1 Overview of Region 4 Introductions Since 2000 ....................................................................................... 1 Format of Species Accounts ...................................................................................................................... 2 Explanation of Maps ................................................................................................................................ -

Natural Resources Technical Report
TRANSFORM 66 OUTSIDE the Beltway I-66 CORRIDOR 66 IMPROVEMENTS PROJECT Multimodal Solutions - 495 to Haymarket Tier 2 Draft Environmental Assessment 193 Town of Natural Resources TechnicalTown of Report Middleburg Herndon LOUDOUN FAUQUIER 50 267 Washington Dulles McLean International Airport 309 28 286 Tysons Corner West Falls Church 7 Chantilly Dunn Loring FALLS 123 CHURCH 29 Vienna LOUDOUN Fair Lakes 50 FAIRFAX CO. 66 15 FAIRFAX CITY Centreville 286 29 236 Manassas National Battlefield Park Haymarket Fairfax Station Springfield 66 Gainesville 234 28 MANASSAS PARK PRINCE WILLIAM 29 FAUQUIER 234 123 286 215 Ft. Belvoir MANASSAS MAY 12, 2015 Tier 2 Draft Environmental Assessment Natural Resources Technical Report Draft – May 12, 2015 I-66 Corridor Improvements Project – Natural Resources Technical Report May 12, 2015 Table of Contents Chapter 1 – Introduction .......................................................................................................................... 1-1 1.1 Project Description ..................................................................................................................... 1-1 1.2 Methods ...................................................................................................................................... 1-2 Chapter 2 – Affected Environment ......................................................................................................... 2-1 2.1 Water Resources ......................................................................................................................