IV.-Organo-Derivatives of Bismuth
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A COORDINATION and REDOX CHEMISTRY of ANTIMONY-BASED LIGANDS and APPLICATIONS in ORGANOMETALLIC CATALYSIS a Dissertation by YING
COORDINATION AND REDOX CHEMISTRY OF ANTIMONY-BASED LIGANDS AND APPLICATIONS IN ORGANOMETALLIC CATALYSIS A Dissertation by YING-HAO LO Submitted to the Office of Graduate and Professional Studies of Texas A&M University in partial fulfillment of the requirements for the degree of DOCTOR OF PHILOSOPHY Chair of Committee, François P. Gabbaï Committee Members, Michael B. Hall Michael Nippe Hung-Jen Wu Head of Department, Simon W. North December 2019 Major Subject: Chemistry Copyright 2019 Ying-Hao Lo a ABSTRACT The use of Z-ligands to modulate the electronic property of transition metal centers is a powerful strategy in catalyst design. Our group has shown that antimony- based ambiphilic ligand, accept electron density from adjacent metal centers by the σ* orbital of the antimony (V) center and thus increasing the electrophilic reactivity of the trancition metal center. In this thesis, we were eager to determine if the charge of the Z- type ligand can be used to further enhance this effect. To this end, we synthesized a 2+ 2+ dicationic gold complex [46] ([(o‐(Ph2P)C6H4)2(o‐Ph2PO)C6H4)SbAuCl] ) featuring a dicationic antimony (V) ligand with a phosphine oxide arm coordinating to the antimony center. Both experimental and computational results show that the gold complex possess a strong Au→Sb interaction reinforced by the dicationic character of the antimony center. The gold‐bound chloride anion of this complex is rather inert and necessitates the addition of excess AgNTf2 to undergo activation. The activated complex, referred to as 2+ 2+ [47] ([((o‐(Ph2P)C6H4)2(o‐Ph2PO)C6H4)SbAuNTf2] ) readily catalyzes both the polymerization and the hydroamination of styrene. -

Polymorphism, Halogen Bonding, and Chalcogen Bonding in the Diiodine Adducts of 1,3- and 1,4-Dithiane
molecules Article Polymorphism, Halogen Bonding, and Chalcogen Bonding in the Diiodine Adducts of 1,3- and 1,4-Dithiane Andrew J. Peloquin 1, Srikar Alapati 2, Colin D. McMillen 1, Timothy W. Hanks 2 and William T. Pennington 1,* 1 Department of Chemistry, Clemson University, Clemson, SC 29634, USA; [email protected] (A.J.P.); [email protected] (C.D.M.) 2 Department of Chemistry, Furman University, Greenville, SC 29613, USA; [email protected] (S.A.); [email protected] (T.W.H.) * Correspondence: [email protected] Abstract: Through variations in reaction solvent and stoichiometry, a series of S-diiodine adducts of 1,3- and 1,4-dithiane were isolated by direct reaction of the dithianes with molecular diiodine in solution. In the case of 1,3-dithiane, variations in reaction solvent yielded both the equatorial and the axial isomers of S-diiodo-1,3-dithiane, and their solution thermodynamics were further studied via DFT. Additionally, S,S’-bis(diiodo)-1,3-dithiane was also isolated. The 1:1 cocrystal, (1,4-dithiane)·(I2) was further isolated, as well as a new polymorph of S,S’-bis(diiodo)-1,4-dithiane. Each structure showed significant S···I halogen and chalcogen bonding interactions. Further, the product of the diiodine-promoted oxidative addition of acetone to 1,4-dithiane, as well as two new cocrystals of 1,4-dithiane-1,4-dioxide involving hydronium, bromide, and tribromide ions, was isolated. Keywords: crystal engineering; chalcogen bonding; halogen bonding; polymorphism; X-ray diffraction Citation: Peloquin, A.J.; Alapati, S.; McMillen, C.D.; Hanks, T.W.; Pennington, W.T. -

Solubility and Solution-Phase Chemistry of Isocyanic Acid, Methyl Isocyanate, 2 and Cyanogen Halides 3 4 5 6 James M
Atmos. Chem. Phys. Discuss., https://doi.org/10.5194/acp-2018-1160 Manuscript under review for journal Atmos. Chem. Phys. Discussion started: 9 November 2018 c Author(s) 2018. CC BY 4.0 License. 1 Solubility and Solution-phase Chemistry of Isocyanic Acid, Methyl Isocyanate, 2 and Cyanogen Halides 3 4 5 6 James M. Roberts1, and Yong Liu2 7 8 1. NOAA/ESRL Chemical Sciences Division, Boulder, Colorado, 80305 9 2. Department of Chemistry, University of Colorado, Denver, Denver, Colorado, 80217 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 Atmos. Chem. Phys. Discuss., https://doi.org/10.5194/acp-2018-1160 Manuscript under review for journal Atmos. Chem. Phys. Discussion started: 9 November 2018 c Author(s) 2018. CC BY 4.0 License. 52 Abstract 53 54 Condensed phase uptake and reaction are important atmospheric removal processes for reduced nitrogen 55 species, isocyanic acid (HNCO), methyl isocyanate (CH3NCO) and cyanogen halides (XCN, X =Cl, Br, I), yet many 56 of the fundamental quantities that govern this chemistry have not been measured or are understudied. Solubilities 57 and first-order reaction rates of these species were measured for a variety of solutions using a bubble flow reactor 58 method with total reactive nitrogen (Nr) detection. The aqueous solubility of HNCO was measured as a function of 59 pH, and exhibited the classic behavior of a weak acid, with an intrinsic Henry’s law solubility of 20 (±2) M/atm, and -4 60 a Ka of 2.0 (±0.28) ×10 M (which corresponds to pKa = 3.7 ±0.06) at 298K. -

Hydroxyacetonitrile (HOCH2CN) As a Precursor for Formylcyanide (CHOCN), Ketenimine (CH2CNH), and Cyanogen (NCCN) in Astrophysical Conditions
A&A 549, A93 (2013) Astronomy DOI: 10.1051/0004-6361/201219779 & c ESO 2013 Astrophysics Hydroxyacetonitrile (HOCH2CN) as a precursor for formylcyanide (CHOCN), ketenimine (CH2CNH), and cyanogen (NCCN) in astrophysical conditions G. Danger1, F. Duvernay1, P. Theulé1, F. Borget1, J.-C. Guillemin2, and T. Chiavassa1 1 Aix-Marseille Univ, CNRS, PIIM UMR 7345, 13397 Marseille, France e-mail: [email protected] 2 Institut des Sciences Chimiques de Rennes, École Nationale Supérieure de Chimie de Rennes, CNRS, UMR 6226, Avenue du Général Leclerc, CS 50837, 35708 Rennes Cedex 7, France Received 8 June 2012 / Accepted 19 November 2012 ABSTRACT Context. The reactivity in astrophysical environments can be investigated in the laboratory through experimental simulations, which provide understanding of the formation of specific molecules detected in the solid phase or in the gas phase of these environments. In this context, the most complex molecules are generally suggested to form at the surface of interstellar grains and to be released into the gas phase through thermal or non-thermal desorption, where they can be detected through rotational spectroscopy. Here, we focus our experiments on the photochemistry of hydroxyacetonitrile (HOCH2CN), whose formation has been shown to compete with aminomethanol (NH2CH2OH), a glycine precursor, through the Strecker synthesis. Aims. We present the first experimental investigation of the ultraviolet (UV) photochemistry of hydroxyacetonitrile (HOCH2CN) as a pure solid or diluted in water ice. Methods. We used Fourier transform infrared (FT-IR) spectroscopy to characterize photoproducts of hydroxyacetonitrile (HOCH2CN) and to determine the different photodegradation pathways of this compound. To improve the photoproduct identifications, irradiations of hydroxyacetonitrile 14N and 15N isotopologues were performed, coupled with theoretical calculations. -
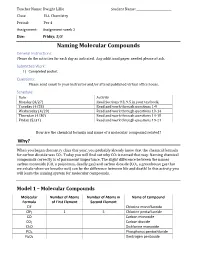
Naming Molecular Compounds General Instructions: Please Do the Activities for Each Day As Indicated
Teacher Name: Dwight Lillie Student Name: ________________________ Class: ELL Chemistry Period: Per 4 Assignment: Assignment week 2 Due: Friday, 5/8 Naming Molecular Compounds General Instructions: Please do the activities for each day as indicated. Any additional paper needed please attach. Submitted Work: 1) Completed packet. Questions: Please send email to your instructor and/or attend published virtual office hours. Schedule: Date Activity Monday (4/27) Read Sections 9.3, 9.5 in your textbook. Tuesday (4/28) Read and work through questions 1-9 Wednesday (4/29) Read and work through questions 10-14 Thursday (4/30) Read and work through questions 14-18 Friday (5/31) Read and work through questions 19-21 How are the chemical formula and name of a molecular compound related? Why? When you began chemistry class this year, you probably already knew that the chemical formula for carbon dioxide was CO2. Today you will find out why CO2 is named that way. Naming chemical compounds correctly is of paramount importance. The slight difference between the names carbon monoxide (CO, a poisonous, deadly gas) and carbon dioxide (CO2, a greenhouse gas that we exhale when we breathe out) can be the difference between life and death! In this activity you will learn the naming system for molecular compounds. Model 1 – Molecular Compounds Molecular Number of Atoms Number of Atoms in Name of Compound Formula of First Element Second Element ClF Chlorine monofluoride ClF5 1 5 Chlorine pentafluoride CO Carbon monoxide CO2 Carbon dioxide Cl2O Dichlorine monoxide PCl5 Phosphorus pentachloride N2O5 Dinitrogen pentoxide 1. Fill in the table to indicate the number of atoms of each type in the molecular formula. -

SAFETY DATA SHEET Nonflammable Gas Mixture: Cyanogen Chloride 1-999Ppm / Nitrogen 99%
SAFETY DATA SHEET Nonflammable Gas Mixture: Cyanogen Chloride 1-999ppm / Nitrogen 99% Section 1. Identification GHS product identifier : Nonflammable Gas Mixture: Cyanogen Chloride 1-999ppm / Nitrogen 99% Other means of : Not available. identification Product use : Synthetic/Analytical chemistry. SDS # : 012226 Supplier's details : Airgas USA, LLC and its affiliates 259 North Radnor-Chester Road Suite 100 Radnor, PA 19087-5283 1-610-687-5253 24-hour telephone : 1-866-734-3438 Section 2. Hazards identification OSHA/HCS status : This material is considered hazardous by the OSHA Hazard Communication Standard (29 CFR 1910.1200). Classification of the : GASES UNDER PRESSURE - Compressed gas substance or mixture GHS label elements Hazard pictograms : Signal word : Warning Hazard statements : Contains gas under pressure; may explode if heated. May displace oxygen and cause rapid suffocation. Precautionary statements General : Read and follow all Safety Data Sheets (SDS’S) before use. Read label before use. Keep out of reach of children. If medical advice is needed, have product container or label at hand. Close valve after each use and when empty. Use equipment rated for cylinder pressure. Do not open valve until connected to equipment prepared for use. Use a back flow preventative device in the piping. Use only equipment of compatible materials of construction. Prevention : Not applicable. Response : Not applicable. Storage : Protect from sunlight when ambient temperature exceeds 52°C/125°F. Store in a well- ventilated place. Disposal : Not applicable. Hazards not otherwise : In addition to any other important health or physical hazards, this product may displace classified oxygen and cause rapid suffocation. Section 3. Composition/information on ingredients Substance/mixture : Mixture Other means of : Not available. -

Cyanogen Metabolism in Cassava Roots: Impact on Protein Synthesis and Root Development
fpls-08-00220 February 22, 2017 Time: 15:3 # 1 View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Frontiers - Publisher Connector ORIGINAL RESEARCH published: 24 February 2017 doi: 10.3389/fpls.2017.00220 Cyanogen Metabolism in Cassava Roots: Impact on Protein Synthesis and Root Development Tawanda Zidenga1*, Dimuth Siritunga2 and Richard T. Sayre1,3 1 Bioscience Division, Los Alamos National Laboratory, Los Alamos, NM, USA, 2 Department of Biology, University of Puerto Rico, Mayaguez, PR, USA, 3 New Mexico Consortium, Los Alamos, NM, USA Cassava (Manihot esculenta Crantz), a staple crop for millions of sub-Saharan Africans, contains high levels of cyanogenic glycosides which protect it against herbivory. However, cyanogens have also been proposed to play a role in nitrogen transport from leaves to roots. Consistent with this hypothesis, analyses of the distribution and activities of enzymes involved in cyanide metabolism provides evidence for cyanide assimilation, derived from linamarin, into amino acids in cassava roots. Both b-cyanoalanine synthase (CAS) and nitrilase (NIT), two enzymes involved in cyanide assimilation to produce asparagine, were observed to have higher activities in roots compared to leaves, consistent with their proposed role in reduced nitrogen assimilation. In addition, rhodanese activity was not detected in cassava roots, indicating that this competing means for cyanide metabolism was not a factor in cyanide detoxification. In contrast, Edited by: Henrik Toft Simonsen, leaves had sufficient rhodanese activity to compete with cyanide assimilation into Technical University of Denmark, amino acids. Using transgenic low cyanogen plants, it was shown that reducing root Denmark cyanogen levels is associated with elevated root nitrate reductase activity, presumably to Reviewed by: compensate for the loss of reduced nitrogen from cyanogens. -

Studies on Halogen-Cyanides Ii . the Distribution of Iodine
Det Kgl . Danske Videnskabernes Selskab. Mathematisk-fysiske Meddelelser. XIV, 3. STUDIES ON HALOGEN-CYANIDES II . THE DISTRIBUTION OF IODINE CYANIDE AN D CYANOGEN BROMIDE BETWEEN BENZENE AN D WATER, AND BETWEEN BENZENE AND SOM E AQUEOUS SALT SOLUTIONS . THE SYNTHESIS AND THE MOLECULAR WEIGHT OF IODIN E CYANID E BY MAX MØLLE R KØBENHAVN LKVIN & MUNKSGAARll EJNAR MUN[fSGAAR D 1936 Printed in Denmark . Bianco Lunos Bogtrykkeri A/S . 1 . Introduction. In an investigation of the equilibrium : ICN+I- +H+ I2, +HCN , KOVACH 1 attempted to calculate the equilibrium constant . But the value which was obtained from the conductivit y measurements and the potentiometric estimations cannot be correct, such as it was realized already by KOVACH , since the value of the iodine-iodide ion potential, calculate d by means of the equilibrium constant, is not in agreemen t with the accepted value . This disagreement may be due to, either that the acti- vity of iodine cyanide is strongly effected by salts in general , or that iodine cyanide reacts with iodide ions and cyanid e ions to form complex compounds, or to both of these effects . The following research was started to investigate th e salting out effect on iodine cyanide and cyanogen bromide , and to investigate if these substances actually are able t o unite with halogen ions to form complex ions, similar to the tri-iodide ion, I . When this investigation was in progress YOST & STONE 2 published a paper dealing with the complexformation of iodine cyanide with iodide and cyanide ions . 1 Z . phys . Chem . 80 (1912) 107 . -

Strategies for Detecting Biological Molecules on Titan
ASTROBIOLOGY Volume 18, Number 5, 2018 ª Mary Ann Liebert, Inc. DOI: 10.1089/ast.2017.1758 Strategies for Detecting Biological Molecules on Titan Catherine D. Neish,1 Ralph D. Lorenz,2 Elizabeth P. Turtle,2 Jason W. Barnes,3 Melissa G. Trainer,4 Bryan Stiles,5 Randolph Kirk,6 Charles A. Hibbitts,2 and Michael J. Malaska5 Abstract Saturn’s moon Titan has all the ingredients needed to produce ‘‘life as we know it.’’ When exposed to liquid water, organic molecules analogous to those found on Titan produce a range of biomolecules such as amino acids. Titan thus provides a natural laboratory for studying the products of prebiotic chemistry. In this work, we examine the ideal locales to search for evidence of, or progression toward, life on Titan. We determine that the best sites to identify biological molecules are deposits of impact melt on the floors of large, fresh impact craters, specifically Sinlap, Selk, and Menrva craters. We find that it is not possible to identify biomolecules on Titan through remote sensing, but rather through in situ measurements capable of identifying a wide range of biological molecules. Given the nonuniformity of impact melt exposures on the floor of a weathered impact crater, the ideal lander would be capable of precision targeting. This would allow it to identify the locations of fresh impact melt deposits, and/or sites where the melt deposits have been exposed through erosion or mass wasting. Determining the extent of prebiotic chemistry within these melt deposits would help us to understand how life could originate on a world very different from Earth. -

Abbreviations of Chemical Warfare Agents, 152 Acetylcholinesterase
Index Abbreviations of chemical warfare agents, Blood ChE inhibition, nerve agent critical 152 toxic effect on, 23 Acetylcholinesterase (AChE) inhibition, Blood ChE inhibition, nerve agent species nerve agents and, 18 variation of, 21 Acetylthiocholine iodide, RBC-ChE activ Blood cholinesterases, nerve agent effects ity and, 22 on, 20 AChE (acetylcholinesterase) inhibition, Butyrylcholinesterase (plasma-ChE), nerve agents and, 18 nerve agent effects on, 20 Acronyms for chemical warfare agents, 152 Acute inhalation toxicity, CK and, 114 Carboxylesterases (aliesterases), nerve Acute toxicity, CK (cyanogen chloride) agent effects on, 21 and, 113 Carcinogenicity, CK and, 122 Acute toxicity, GA and, 68 Carcinogenicity, GA and, 73 Acute toxicity, GB and, 78 Carcinogenicity, GB and, 90 Acute toxicity, GD and, 94 Carcinogenicity, HD and, 38 Acute toxicity, HD and, 30 Carcinogenicity, HN2 and, 53 Acute toxicity, HN2 and, 51 Carcinogenicity, HT and, 49 Acute toxicity, HT and, 49 Carcinogenicity, lewisite and, 107 Acute toxicity, lewisite and, 103 Carcinogenicity, VX and, 61 Acute toxicity, T sulfur mustard and, 50 ChE (cholinesterase), nerve agent effects Acute toxicity, VX and, 54 on, 20 Agent CK, see CK or cyanogen chloride ChE inhibition, nerve agent potency on, Agent GA, see GA or Tabun 23 Agent GB, see GB or Sarin Chemical warfare agent disposal tech Agent GD, see GD or Soman niques,3 Agent HD, see HD or Sulfur mustard HD Chemical warfare agent non-stockpile Agent HN2, see HN2 or Nitrogen mus- sites U.S., 4 tard Chemical warfare agents, 1 ff. Agent HT, see HT or Sulfur mustard HT Chemical warfare agents, acronyms/abbre Agent L, see L or Lewsite viations of, 152 Agent T, see T Sulfur mustard Chemical warfare agents, air exposure lim Agent VX, see VX its of, 7 Aliesterases (carboxylesterases), nerve Chemical warfare agents, environmental agent effects on, 21 cleanup of, 3 Anticholinesterase agents, 18 Chemical warfare agents, environmental Army chemical destruction, 3 ff. -

Pp-03-25-New Dots.Qxd 10/23/02 2:16 PM Page 136
pp-03-25-new dots.qxd 10/23/02 2:16 PM Page 136 136 BROMIC ACID / BROMINE BROMIC ACID [7789–31–3] Formula: HBrO3; MW 128.91 Uses Bromic acid is used as an oxidizing agent; and also as intermediate in the preparation of dyes and pharmaceuticals . Physical Properties Unstable compound; stable only in dilute aqueous solutions; solution turns yellow on standing; decomposes when heated to 100°C. Preparation Bromic acid is prepared by adding sulfuric acid to barium bromate. Ba(BrO3)2 + H2SO4 → 2HBrO3 + BaSO4 The product is distilled and absorbed in water. A 50% solution may be obtained by slow evaporation of the dilute solution in vacuum at –12°C. Toxicity Contact with skin and eyes can cause severe irritation. BROMINE [7726–95–6] Symbol Br; atomic number 35; atomic weight 79.904; a halogen group ele- ment; electron affinity 3.36359 eV; electronegativity 2.8; electron configura- tion [Ar] 3d104s24p5; most stable valence states –1 and +5, less stable valence states +1 and +3; a diatomic molecule (Br2) in liquid and vapor states over a wide range of temperature; two stable isotopes, Br–79 (50.57%) and Br–81 (49.43%). Occurrence and Uses Bromine occurs in nature as bromide in many natural brine wells and salt deposits. It also is found in seawater at a concentration of 85 mg/L. The ele- ment was discovered by A. J. Balard and C. Lowig, independently in 1826. Bromine is used in bleaching fibers and as a disinfectant for water purifica- tion. Other applications are in organic synthesis as an oxidizing or brominat- ing agent; in the manufacture of ethylene dibromide, methyl bromide and other bromo compounds for dyes and pharmaceutical uses; as a fire retardant for plastics; and in chemical analysis. -

United States Patent (19) 11) 4,183,924 Green Et Al
United States Patent (19) 11) 4,183,924 Green et al. 45 Jan. 15, 1980 (54) 17a-CHLORO-179-HYDROCARBONSULFI 58) Field of Search .................... 260/239.55 R, 397.3, NYL-1,4-ANDROSTADENES AND THE 260/.397.45; 424/242; Steroids MS File/ CORRESPONDING 176-HYDROCARBONSULFONYL 56 References Cited DERVATIVES AND THEIR USE AS U.S. PATENT DOCUMENTS ANT-ACNE AGENTS 4,091,036 5/1978 Varma ........................... 260/.397.3 X 75 Inventors: Michael J. Green, Kendall Park; Primary Examiner-Ethel G. Love Robert Tiberi, Englishtown, both of Attorney, Agent, or Firm-Elizabeth A. Bellamy; Mary N.J. S. King 73 Assignee: Schering Corporation, Kenilworth, N.J. 57 ABSTRACT Novel 17a-chloro-1743-hydrocarbonsulfinyl and 17a 21) Appl. No.: 881,217 chloro-17 (3-hydrocarbonsulfonyl derivatives of 3-oxo (22 Filed: Feb. 27, 1978 1,4-androstadienes and their use as anti-acne agents are 51) Int. C.? ...................... A61K 31/565; C07J 31/00 described. 52 U.S.C. ........................... 424/242; 260/239.55 R; 260/.397.3; 260/.397.45 18 Claims, No Drawings 4,183,924 1. 2 Preferred compounds of our invention are those de 17a-CHLORO-176-HYDROCARBONSULFINYL fined by the following formula II: 1,4-ANDROSTADIENES AND THE CORRESPONDING II 176-HYDROCARBONSULFONYL DERIVATIVES 5 AND THER USE AS ANT-ACNE AGENTS FIELD OF THE INVENTION This invention relates to novel compositions-of-mat ter, to methods for their manufacture and methods for 10 their use as anti-acne agents. Specifically, this invention relates to novel 17a chloro-176-hydrocarbonsulfinyl and 17a-chloro-1718 hydrocarbonsulfonyl derivatives of 3-oxo-1,4-andros 15 tadienes useful as anti-acne agents.