Chemical Potential and Gibbs Distribution
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Thermodynamic Potentials and Natural Variables
Revista Brasileira de Ensino de Física, vol. 42, e20190127 (2020) Articles www.scielo.br/rbef cb DOI: http://dx.doi.org/10.1590/1806-9126-RBEF-2019-0127 Licença Creative Commons Thermodynamic Potentials and Natural Variables M. Amaku1,2, F. A. B. Coutinho*1, L. N. Oliveira3 1Universidade de São Paulo, Faculdade de Medicina, São Paulo, SP, Brasil 2Universidade de São Paulo, Faculdade de Medicina Veterinária e Zootecnia, São Paulo, SP, Brasil 3Universidade de São Paulo, Instituto de Física de São Carlos, São Carlos, SP, Brasil Received on May 30, 2019. Revised on September 13, 2018. Accepted on October 4, 2019. Most books on Thermodynamics explain what thermodynamic potentials are and how conveniently they describe the properties of physical systems. Certain books add that, to be useful, the thermodynamic potentials must be expressed in their “natural variables”. Here we show that, given a set of physical variables, an appropriate thermodynamic potential can always be defined, which contains all the thermodynamic information about the system. We adopt various perspectives to discuss this point, which to the best of our knowledge has not been clearly presented in the literature. Keywords: Thermodynamic Potentials, natural variables, Legendre transforms. 1. Introduction same statement cannot be applied to the temperature. In real fluids, even in simple ones, the proportionality Basic concepts are most easily understood when we dis- to T is washed out, and the Internal Energy is more cuss simple systems. Consider an ideal gas in a cylinder. conveniently expressed as a function of the entropy and The cylinder is closed, its walls are conducting, and a volume: U = U(S, V ). -

Thermodynamics Notes
Thermodynamics Notes Steven K. Krueger Department of Atmospheric Sciences, University of Utah August 2020 Contents 1 Introduction 1 1.1 What is thermodynamics? . .1 1.2 The atmosphere . .1 2 The Equation of State 1 2.1 State variables . .1 2.2 Charles' Law and absolute temperature . .2 2.3 Boyle's Law . .3 2.4 Equation of state of an ideal gas . .3 2.5 Mixtures of gases . .4 2.6 Ideal gas law: molecular viewpoint . .6 3 Conservation of Energy 8 3.1 Conservation of energy in mechanics . .8 3.2 Conservation of energy: A system of point masses . .8 3.3 Kinetic energy exchange in molecular collisions . .9 3.4 Working and Heating . .9 4 The Principles of Thermodynamics 11 4.1 Conservation of energy and the first law of thermodynamics . 11 4.1.1 Conservation of energy . 11 4.1.2 The first law of thermodynamics . 11 4.1.3 Work . 12 4.1.4 Energy transferred by heating . 13 4.2 Quantity of energy transferred by heating . 14 4.3 The first law of thermodynamics for an ideal gas . 15 4.4 Applications of the first law . 16 4.4.1 Isothermal process . 16 4.4.2 Isobaric process . 17 4.4.3 Isosteric process . 18 4.5 Adiabatic processes . 18 5 The Thermodynamics of Water Vapor and Moist Air 21 5.1 Thermal properties of water substance . 21 5.2 Equation of state of moist air . 21 5.3 Mixing ratio . 22 5.4 Moisture variables . 22 5.5 Changes of phase and latent heats . -

PDF Version of Helmholtz Free Energy
Free energy Free Energy at Constant T and V Starting with the First Law dU = δw + δq At constant temperature and volume we have δw = 0 and dU = δq Free Energy at Constant T and V Starting with the First Law dU = δw + δq At constant temperature and volume we have δw = 0 and dU = δq Recall that dS ≥ δq/T so we have dU ≤ TdS Free Energy at Constant T and V Starting with the First Law dU = δw + δq At constant temperature and volume we have δw = 0 and dU = δq Recall that dS ≥ δq/T so we have dU ≤ TdS which leads to dU - TdS ≤ 0 Since T and V are constant we can write this as d(U - TS) ≤ 0 The quantity in parentheses is a measure of the spontaneity of the system that depends on known state functions. Definition of Helmholtz Free Energy We define a new state function: A = U -TS such that dA ≤ 0. We call A the Helmholtz free energy. At constant T and V the Helmholtz free energy will decrease until all possible spontaneous processes have occurred. At that point the system will be in equilibrium. The condition for equilibrium is dA = 0. A time Definition of Helmholtz Free Energy Expressing the change in the Helmholtz free energy we have ∆A = ∆U – T∆S for an isothermal change from one state to another. The condition for spontaneous change is that ∆A is less than zero and the condition for equilibrium is that ∆A = 0. We write ∆A = ∆U – T∆S ≤ 0 (at constant T and V) Definition of Helmholtz Free Energy Expressing the change in the Helmholtz free energy we have ∆A = ∆U – T∆S for an isothermal change from one state to another. -

Chapter 3. Second and Third Law of Thermodynamics
Chapter 3. Second and third law of thermodynamics Important Concepts Review Entropy; Gibbs Free Energy • Entropy (S) – definitions Law of Corresponding States (ch 1 notes) • Entropy changes in reversible and Reduced pressure, temperatures, volumes irreversible processes • Entropy of mixing of ideal gases • 2nd law of thermodynamics • 3rd law of thermodynamics Math • Free energy Numerical integration by computer • Maxwell relations (Trapezoidal integration • Dependence of free energy on P, V, T https://en.wikipedia.org/wiki/Trapezoidal_rule) • Thermodynamic functions of mixtures Properties of partial differential equations • Partial molar quantities and chemical Rules for inequalities potential Major Concept Review • Adiabats vs. isotherms p1V1 p2V2 • Sign convention for work and heat w done on c=C /R vm system, q supplied to system : + p1V1 p2V2 =Cp/CV w done by system, q removed from system : c c V1T1 V2T2 - • Joule-Thomson expansion (DH=0); • State variables depend on final & initial state; not Joule-Thomson coefficient, inversion path. temperature • Reversible change occurs in series of equilibrium V states T TT V P p • Adiabatic q = 0; Isothermal DT = 0 H CP • Equations of state for enthalpy, H and internal • Formation reaction; enthalpies of energy, U reaction, Hess’s Law; other changes D rxn H iD f Hi i T D rxn H Drxn Href DrxnCpdT Tref • Calorimetry Spontaneous and Nonspontaneous Changes First Law: when one form of energy is converted to another, the total energy in universe is conserved. • Does not give any other restriction on a process • But many processes have a natural direction Examples • gas expands into a vacuum; not the reverse • can burn paper; can't unburn paper • heat never flows spontaneously from cold to hot These changes are called nonspontaneous changes. -

Thermodynamics, Flame Temperature and Equilibrium
Thermodynamics, Flame Temperature and Equilibrium Combustion Summer School 2018 Prof. Dr.-Ing. Heinz Pitsch Course Overview Part I: Fundamentals and Laminar Flames • Introduction • Fundamentals and mass balances of combustion systems • Thermodynamic quantities • Thermodynamics, flame • Flame temperature at complete temperature, and equilibrium conversion • Governing equations • Chemical equilibrium • Laminar premixed flames: Kinematics and burning velocity • Laminar premixed flames: Flame structure • Laminar diffusion flames • FlameMaster flame calculator 2 Thermodynamic Quantities First law of thermodynamics - balance between different forms of energy • Change of specific internal energy: du specific work due to volumetric changes: δw = -pdv , v=1/ρ specific heat transfer from the surroundings: δq • Related quantities specific enthalpy (general definition): specific enthalpy for an ideal gas: • Energy balance for a closed system: 3 Multicomponent system • Specific internal energy and specific enthalpy of mixtures • Relation between internal energy and enthalpy of single species 4 Multicomponent system • Ideal gas u and h only function of temperature • If cpi is specific heat at constant pressure and hi,ref is reference enthalpy at reference temperature Tref , temperature dependence of partial specific enthalpy is given by • Reference temperature may be arbitrarily chosen, most frequently used: Tref = 0 K or Tref = 298.15 K 5 Multicomponent system • Partial molar enthalpy hi,m is and its temperature dependence is where the molar specific -

Particle Characterisation In
LIFE SCIENCE I TECHNICAL BULLETIN ISSUE N°11 /JULY 2008 PARTICLE CHARACTERISATION IN EXCIPIENTS, DRUG PRODUCTS AND DRUG SUBSTANCES AUTHOR: HILDEGARD BRÜMMER, PhD, CUSTOMER SERVICE MANAGER, SGS LIFE SCIENCE SERVICES, GERMANY Particle characterization has significance in many industries. For the pharmaceutical industry, particle size impacts products in two ways: as an influence on drug performance and as an indicator of contamination. This article will briefly examine how particle size impacts both, and review the arsenal of methods for measuring and tracking particle size. Furthermore, examples of compromised product quality observed within our laboratories illustrate why characterization is so important. INDICATOR OF CONTAMINATION Controlling the limits of contaminating • Adverse indirect reactions: particles Particle characterisation of drug particles is critical for injectable are identified by the immune system as substances, drug products and excipients (parenteral) solutions. Particle foreign material and immune reaction is an important factor in R&D, production contamination of solutions can potentially might impose secondary effects. and quality control of pharmaceuticals. have the following results: It is becoming increasingly important for In order to protect a patient and to compliance with requirements of FDA and • Adverse direct reactions: e.g. particles guarantee a high quality product, several European Health Authorities. are distributed via the blood in the body chapters in the compendia (USP, EP, JP) and cause toxicity to specific tissues or describe techniques for characterisation organs, or particles of a given size can of limits. Some of the most relevant cause a physiological effect blocking blood chapters are listed in Table 1. flow e.g. in the lungs. -

Chapter 3 Bose-Einstein Condensation of an Ideal
Chapter 3 Bose-Einstein Condensation of An Ideal Gas An ideal gas consisting of non-interacting Bose particles is a ¯ctitious system since every realistic Bose gas shows some level of particle-particle interaction. Nevertheless, such a mathematical model provides the simplest example for the realization of Bose-Einstein condensation. This simple model, ¯rst studied by A. Einstein [1], correctly describes important basic properties of actual non-ideal (interacting) Bose gas. In particular, such basic concepts as BEC critical temperature Tc (or critical particle density nc), condensate fraction N0=N and the dimensionality issue will be obtained. 3.1 The ideal Bose gas in the canonical and grand canonical ensemble Suppose an ideal gas of non-interacting particles with ¯xed particle number N is trapped in a box with a volume V and at equilibrium temperature T . We assume a particle system somehow establishes an equilibrium temperature in spite of the absence of interaction. Such a system can be characterized by the thermodynamic partition function of canonical ensemble X Z = e¡¯ER ; (3.1) R where R stands for a macroscopic state of the gas and is uniquely speci¯ed by the occupa- tion number ni of each single particle state i: fn0; n1; ¢ ¢ ¢ ¢ ¢ ¢g. ¯ = 1=kBT is a temperature parameter. Then, the total energy of a macroscopic state R is given by only the kinetic energy: X ER = "ini; (3.2) i where "i is the eigen-energy of the single particle state i and the occupation number ni satis¯es the normalization condition X N = ni: (3.3) i 1 The probability -

Thermodynamics
ME346A Introduction to Statistical Mechanics { Wei Cai { Stanford University { Win 2011 Handout 6. Thermodynamics January 26, 2011 Contents 1 Laws of thermodynamics 2 1.1 The zeroth law . .3 1.2 The first law . .4 1.3 The second law . .5 1.3.1 Efficiency of Carnot engine . .5 1.3.2 Alternative statements of the second law . .7 1.4 The third law . .8 2 Mathematics of thermodynamics 9 2.1 Equation of state . .9 2.2 Gibbs-Duhem relation . 11 2.2.1 Homogeneous function . 11 2.2.2 Virial theorem / Euler theorem . 12 2.3 Maxwell relations . 13 2.4 Legendre transform . 15 2.5 Thermodynamic potentials . 16 3 Worked examples 21 3.1 Thermodynamic potentials and Maxwell's relation . 21 3.2 Properties of ideal gas . 24 3.3 Gas expansion . 28 4 Irreversible processes 32 4.1 Entropy and irreversibility . 32 4.2 Variational statement of second law . 32 1 In the 1st lecture, we will discuss the concepts of thermodynamics, namely its 4 laws. The most important concepts are the second law and the notion of Entropy. (reading assignment: Reif x 3.10, 3.11) In the 2nd lecture, We will discuss the mathematics of thermodynamics, i.e. the machinery to make quantitative predictions. We will deal with partial derivatives and Legendre transforms. (reading assignment: Reif x 4.1-4.7, 5.1-5.12) 1 Laws of thermodynamics Thermodynamics is a branch of science connected with the nature of heat and its conver- sion to mechanical, electrical and chemical energy. (The Webster pocket dictionary defines, Thermodynamics: physics of heat.) Historically, it grew out of efforts to construct more efficient heat engines | devices for ex- tracting useful work from expanding hot gases (http://www.answers.com/thermodynamics). -

Guide for the Use of the International System of Units (SI)
Guide for the Use of the International System of Units (SI) m kg s cd SI mol K A NIST Special Publication 811 2008 Edition Ambler Thompson and Barry N. Taylor NIST Special Publication 811 2008 Edition Guide for the Use of the International System of Units (SI) Ambler Thompson Technology Services and Barry N. Taylor Physics Laboratory National Institute of Standards and Technology Gaithersburg, MD 20899 (Supersedes NIST Special Publication 811, 1995 Edition, April 1995) March 2008 U.S. Department of Commerce Carlos M. Gutierrez, Secretary National Institute of Standards and Technology James M. Turner, Acting Director National Institute of Standards and Technology Special Publication 811, 2008 Edition (Supersedes NIST Special Publication 811, April 1995 Edition) Natl. Inst. Stand. Technol. Spec. Publ. 811, 2008 Ed., 85 pages (March 2008; 2nd printing November 2008) CODEN: NSPUE3 Note on 2nd printing: This 2nd printing dated November 2008 of NIST SP811 corrects a number of minor typographical errors present in the 1st printing dated March 2008. Guide for the Use of the International System of Units (SI) Preface The International System of Units, universally abbreviated SI (from the French Le Système International d’Unités), is the modern metric system of measurement. Long the dominant measurement system used in science, the SI is becoming the dominant measurement system used in international commerce. The Omnibus Trade and Competitiveness Act of August 1988 [Public Law (PL) 100-418] changed the name of the National Bureau of Standards (NBS) to the National Institute of Standards and Technology (NIST) and gave to NIST the added task of helping U.S. -

Thermodynamic Analysis Based on the Second-Order Variations of Thermodynamic Potentials
Theoret. Appl. Mech., Vol.35, No.1-3, pp. 215{234, Belgrade 2008 Thermodynamic analysis based on the second-order variations of thermodynamic potentials Vlado A. Lubarda ¤ Abstract An analysis of the Gibbs conditions of stable thermodynamic equi- librium, based on the constrained minimization of the four fundamen- tal thermodynamic potentials, is presented with a particular attention given to the previously unexplored connections between the second- order variations of thermodynamic potentials. These connections are used to establish the convexity properties of all potentials in relation to each other, which systematically deliver thermodynamic relationships between the speci¯c heats, and the isentropic and isothermal bulk mod- uli and compressibilities. The comparison with the classical derivation is then given. Keywords: Gibbs conditions, internal energy, second-order variations, speci¯c heats, thermodynamic potentials 1 Introduction The Gibbs conditions of thermodynamic equilibrium are of great importance in the analysis of the equilibrium and stability of homogeneous and heterogeneous thermodynamic systems [1]. The system is in a thermodynamic equilibrium if its state variables do not spontaneously change with time. As a consequence of the second law of thermodynamics, the equilibrium state of an isolated sys- tem at constant volume and internal energy is the state with the maximum value of the total entropy. Alternatively, among all neighboring states with the ¤Department of Mechanical and Aerospace Engineering, University of California, San Diego; La Jolla, CA 92093-0411, USA and Montenegrin Academy of Sciences and Arts, Rista Stijovi¶ca5, 81000 Podgorica, Montenegro, e-mail: [email protected]; [email protected] 215 216 Vlado A. Lubarda same volume and total entropy, the equilibrium state is one with the lowest total internal energy. -

2. Classical Gases
2. Classical Gases Our goal in this section is to use the techniques of statistical mechanics to describe the dynamics of the simplest system: a gas. This means a bunch of particles, flying around in a box. Although much of the last section was formulated in the language of quantum mechanics, here we will revert back to classical mechanics. Nonetheless, a recurrent theme will be that the quantum world is never far behind: we’ll see several puzzles, both theoretical and experimental, which can only truly be resolved by turning on ~. 2.1 The Classical Partition Function For most of this section we will work in the canonical ensemble. We start by reformu- lating the idea of a partition function in classical mechanics. We’ll consider a simple system – a single particle of mass m moving in three dimensions in a potential V (~q ). The classical Hamiltonian of the system3 is the sum of kinetic and potential energy, p~ 2 H = + V (~q ) 2m We earlier defined the partition function (1.21) to be the sum over all quantum states of the system. Here we want to do something similar. In classical mechanics, the state of a system is determined by a point in phase space.Wemustspecifyboththeposition and momentum of each of the particles — only then do we have enough information to figure out what the system will do for all times in the future. This motivates the definition of the partition function for a single classical particle as the integration over phase space, 1 3 3 βH(p,q) Z = d qd pe− (2.1) 1 h3 Z The only slightly odd thing is the factor of 1/h3 that sits out front. -
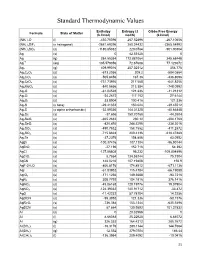
Standard Thermodynamic Values
Standard Thermodynamic Values Enthalpy Entropy (J Gibbs Free Energy Formula State of Matter (kJ/mol) mol/K) (kJ/mol) (NH4)2O (l) -430.70096 267.52496 -267.10656 (NH4)2SiF6 (s hexagonal) -2681.69296 280.24432 -2365.54992 (NH4)2SO4 (s) -1180.85032 220.0784 -901.90304 Ag (s) 0 42.55128 0 Ag (g) 284.55384 172.887064 245.68448 Ag+1 (aq) 105.579056 72.67608 77.123672 Ag2 (g) 409.99016 257.02312 358.778 Ag2C2O4 (s) -673.2056 209.2 -584.0864 Ag2CO3 (s) -505.8456 167.36 -436.8096 Ag2CrO4 (s) -731.73976 217.568 -641.8256 Ag2MoO4 (s) -840.5656 213.384 -748.0992 Ag2O (s) -31.04528 121.336 -11.21312 Ag2O2 (s) -24.2672 117.152 27.6144 Ag2O3 (s) 33.8904 100.416 121.336 Ag2S (s beta) -29.41352 150.624 -39.45512 Ag2S (s alpha orthorhombic) -32.59336 144.01328 -40.66848 Ag2Se (s) -37.656 150.70768 -44.3504 Ag2SeO3 (s) -365.2632 230.12 -304.1768 Ag2SeO4 (s) -420.492 248.5296 -334.3016 Ag2SO3 (s) -490.7832 158.1552 -411.2872 Ag2SO4 (s) -715.8824 200.4136 -618.47888 Ag2Te (s) -37.2376 154.808 43.0952 AgBr (s) -100.37416 107.1104 -96.90144 AgBrO3 (s) -27.196 152.716 54.392 AgCl (s) -127.06808 96.232 -109.804896 AgClO2 (s) 8.7864 134.55744 75.7304 AgCN (s) 146.0216 107.19408 156.9 AgF•2H2O (s) -800.8176 174.8912 -671.1136 AgI (s) -61.83952 115.4784 -66.19088 AgIO3 (s) -171.1256 149.3688 -93.7216 AgN3 (s) 308.7792 104.1816 376.1416 AgNO2 (s) -45.06168 128.19776 19.07904 AgNO3 (s) -124.39032 140.91712 -33.472 AgO (s) -11.42232 57.78104 14.2256 AgOCN (s) -95.3952 121.336 -58.1576 AgReO4 (s) -736.384 153.1344 -635.5496 AgSCN (s) 87.864 130.9592 101.37832 Al (s)