Recent Progress in Laser Spectroscopy of the Actinides
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Taming of “49” Big Science in Little Time
The Taming of “49” Big science in little time Recollections of Edward F. Hammel During the Manhattan Project, plutonium was often referred to, simply, as 49. Number 4 was for the last digit in 94 (the atomic number of plutonium) and 9 for the last digit in plutonium-239, the isotope of choice for nuclear weapons. The story that unfolds was adapted from Plutonium Metallurgy at Los Alamos, 1943–1945, as Edward F. Hammel remembers the events of those years. 48 Los Alamos Science Number 26 2000 The Taming of “49” he work in plutonium chemistry tion work was an inevitable conse- the metal could be fabricated into and metallurgy carried out at quence of the nuclear and physical satisfactory weapon components. TLos Alamos (Site Y) between research that was still to be conducted In addition, not until January 1944 1943 and 1945 had a somewhat contro- on the metal. It would clearly have did the first few milligrams of pile- versial history. The controversy was been inefficient and time consuming to produced plutonium arrive at Los about who was going to do what. ship small amounts of plutonium metal Alamos. The first 1-gram shipment At the time Los Alamos was being back to Chicago for repurification and arrived in February 1944, and quantity organized, most of the expertise in plu- refabrication into different sizes and shipments of plutonium did not begin to tonium chemistry resided at Berkeley, shapes for the next-scheduled nuclear arrive at Los Alamos until May 1945. where plutonium was discovered in physics experiment. From the outset, it was clear that the December 1940, and at the Met Lab in Minimizing the time spent to solve purification of plutonium was the most Chicago. -
Laser Isotope Separation (LIS), Technical and Economic
NASA TECHNICAL MEMORANDUM A STATUS OF PROGRESS FOR THL LASER lsofopE SEPARATION (11 SI PROCESS +tear 1976 NASA George C. Mdr~bdlSpace Flight Center Marshdl Space Fb$t Center, Alabama lLSFC - Form 3190 (Rev June 1971) REPORT STANDARD TITLE PACE I nEPMTn0. 3. RECIPIENT*$ CATILOC NO. NASA TM X-73345 10 TITLE UO SUTlTLt IS. REPORT DATE I September A st.tUaof for Iaser isotOpe ¶tian lS76 I Progress the (LIS) 6 PERFWYIIIG WGUIZATIO* CQOE George C. M8ralmll!3gam Flight Center I 1. COUTRUT OR am yo. I MarW Flight Center, Alabama 35812 Tecbnid Memormdum National Aemutics and Space Administration Washingtan, D.C. 20546 I I Prepared by Systems Aaalysis and Integration Iaboratory, Science and Engineering An overview of the various categories of the LE3 methodology is given together with illustrations showing a simplified version of the LIS tecbnique, an example of the two-phoiin photoionization category, and a diagram depicting how the energy levels of various isdope influence the LIS process. A&icatlons have been proposed for the LIS system which, in addition to the use to enrich uranium, could in themselves develop into programs of tremendous scope and breadth. Such applications as treatment of radioac '--ewastes from light-water nKzlear reactors, enriching the deuterlum isotope to make heavv-water, and enrlchhg tik light isotopes of such 17 KEt WORDS 18. DISTRIBUTION STATEMENT 5ECUQlTY CLASSIF. Ff thh PI*) 21 NO. OF PAbFS 22 PRICE Unclassified Unclassified I 20 NTIS PREFACE Since the publication of t& first Techid hiemomxitun (TM X-64947) on the Laser hotope Separation (LE)process in May 1975 [l], there bbeen a virtual explosion of available information on this process. -

Extensive Interest in Nuclear Fuel Cycle Technologies
Institute for Science and International Security ISIS REPORT March 19, 2012 Department 70 and the Physics Research Center: Extensive Interest in Nuclear Fuel Cycle Technologies By David Albright, Paul Brannan, Mark Gorwitz, and Andrew Ortendahl On February 23, 2012, ISIS released the report, The Physics Research Center and Iran’s Parallel Military Nuclear Program, in which ISIS evaluated a set of 1,600 telexes outlining a set of departments or buying centers of the former Physics Research Center (PHRC). These departments appeared to be purchasing a variety of goods for specific nuclear technologies, including gas centrifuges, uranium conversion, uranium exploration and perhaps mining, and heavy water production. Figure 1 is a list of the purposes of these departments. The telexes are evaluated in more depth in the February 23, 2012 ISIS report and support that, contrary to Iran’s statements to the International Atomic Energy Agency (IAEA), the PHRC ran a parallel military nuclear program in the 1990s. In the telexes, ISIS identified a department called Department 70 that is linked to the PHRC. This department tried to procure or obtained technical publications and reports from a document center, relevant know-how from suppliers, catalogues from suppliers about particular goods, and a mini- computer from the Digital Equipment Corporation. Department 70 appears to have had personnel highly knowledgeable about the existing literature on a variety of fuel cycle technologies, particularly gas centrifuges. Orders to a British document center reveal many technical publications about gas centrifuges, atomic laser isotope enrichment, the production of uranium compounds including uranium tetrafluoride and uranium hexafluoride (and precursors such as hydrofluoric acid), nuclear grade graphite, and the production of heavy water. -

The Development of the Periodic Table and Its Consequences Citation: J
Firenze University Press www.fupress.com/substantia The Development of the Periodic Table and its Consequences Citation: J. Emsley (2019) The Devel- opment of the Periodic Table and its Consequences. Substantia 3(2) Suppl. 5: 15-27. doi: 10.13128/Substantia-297 John Emsley Copyright: © 2019 J. Emsley. This is Alameda Lodge, 23a Alameda Road, Ampthill, MK45 2LA, UK an open access, peer-reviewed article E-mail: [email protected] published by Firenze University Press (http://www.fupress.com/substantia) and distributed under the terms of the Abstract. Chemistry is fortunate among the sciences in having an icon that is instant- Creative Commons Attribution License, ly recognisable around the world: the periodic table. The United Nations has deemed which permits unrestricted use, distri- 2019 to be the International Year of the Periodic Table, in commemoration of the 150th bution, and reproduction in any medi- anniversary of the first paper in which it appeared. That had been written by a Russian um, provided the original author and chemist, Dmitri Mendeleev, and was published in May 1869. Since then, there have source are credited. been many versions of the table, but one format has come to be the most widely used Data Availability Statement: All rel- and is to be seen everywhere. The route to this preferred form of the table makes an evant data are within the paper and its interesting story. Supporting Information files. Keywords. Periodic table, Mendeleev, Newlands, Deming, Seaborg. Competing Interests: The Author(s) declare(s) no conflict of interest. INTRODUCTION There are hundreds of periodic tables but the one that is widely repro- duced has the approval of the International Union of Pure and Applied Chemistry (IUPAC) and is shown in Fig.1. -

Evolution of Intrinsic Nuclear Structure in Medium Mass Even-Even Xenon Isotopes from a Microscopic Perspective*
Chinese Physics C Vol. 44, No. 7 (2020) 074108 Evolution of intrinsic nuclear structure in medium mass even-even Xenon isotopes from a microscopic perspective* Surbhi Gupta1 Ridham Bakshi1 Suram Singh2 Arun Bharti1;1) G. H. Bhat3 J. A. Sheikh4 1Department of Physics, University of Jammu, Jammu- 180006, India 2Department of Physics and Astronomical Sciences, Central University of Jammu, Samba- 181143, India 3Department of Physics, SP College, Cluster University Srinagar- 190001, India 4Cluster University Srinagar - Jammu and Kashmir 190001, India Abstract: In this study, the multi-quasiparticle triaxial projected shell model (TPSM) is applied to investigate γ-vi- brational bands in transitional nuclei of 118−128Xe. We report that each triaxial intrinsic state has a γ-band built on it. The TPSM approach is evaluated by the comparison of TPSM results with available experimental data, which shows a satisfactory agreement. The energy ratios, B(E2) transition rates, and signature splitting of the γ-vibrational band are calculated. Keywords: triaxial projected shell model, triaxiality, yrast spectra, band diagram, back-bending, staggering, reduced transition probabilities DOI: 10.1088/1674-1137/44/7/074108 1 Introduction In the past few decades, the use of improved and sophisticated experimental techniques has made it pos- sible to provide sufficient data to describe the structure of The static and dynamic properties of a nucleus pre- nuclei in various mass regions of the nuclear chart. In dominantly dictate its shape or structure, and these prop- particular, the transitional nuclei around mass A ∼ 130 erties depend on the interactions among its constituents, have numerous interesting features, such as odd-even i.e, protons and neutrons. -

Development of a Viable Route for Lithium-6 Supply of DEMO and Future Fusion Power Plants T
Fusion Engineering and Design 149 (2019) 111339 Contents lists available at ScienceDirect Fusion Engineering and Design journal homepage: www.elsevier.com/locate/fusengdes Development of a viable route for lithium-6 supply of DEMO and future fusion power plants T T. Giegerich⁎, K. Battes, J.C. Schwenzer, C. Day Karlsruhe Institute of Technology (KIT), Hermann-von-Helmholtz-Platz 1, 76344 Eggenstein-Leopoldshafen, Germany ARTICLE INFO ABSTRACT Keywords: In the European DEMO program, the design development of a demonstration power plant (DEMO) is currently in DEMO its pre-conceptual phase. In DEMO, breeding blankets will use large quantities of lithium, enriched in the isotope Lithium-6 lithium-6 (6Li), for breeding the tritium needed to feed the DT fusion reaction. Unfortunately, enriched lithium is Lithium enrichment commercially not available in the required quantities, which is threatening the success of future power plant ICOMAX process applications of nuclear fusion. Even if the manufacturing of the breeding blankets is still two decades ahead of Mercury us, it is now mandatory to address the topic of lithium-6 supply and to make sure that a viable supply (and HgLab reprocessing) route is available when needed. This paper presents an unbiased systems engineering approach assessing a number of available lithium iso- tope separation methods by defining requirements, rating them systematically and finally calculating a ranking number expressing the value of different methods. As a result, we suggest using a chemical exchange method based on a lithium amalgam system, but including some important improvements leading to a more efficient and ‘clean’ process (the ICOMAX process) in comparison with the formerly used COLEX process. -

Quest for Superheavy Nuclei Began in the 1940S with the Syn Time It Takes for Half of the Sample to Decay
FEATURES Quest for superheavy nuclei 2 P.H. Heenen l and W Nazarewicz -4 IService de Physique Nucleaire Theorique, U.L.B.-C.P.229, B-1050 Brussels, Belgium 2Department ofPhysics, University ofTennessee, Knoxville, Tennessee 37996 3Physics Division, Oak Ridge National Laboratory, Oak Ridge, Tennessee 37831 4Institute ofTheoretical Physics, University ofWarsaw, ul. Ho\.za 69, PL-OO-681 Warsaw, Poland he discovery of new superheavy nuclei has brought much The superheavy elements mark the limit of nuclear mass and T excitement to the atomic and nuclear physics communities. charge; they inhabit the upper right corner of the nuclear land Hopes of finding regions of long-lived superheavy nuclei, pre scape, but the borderlines of their territory are unknown. The dicted in the early 1960s, have reemerged. Why is this search so stability ofthe superheavy elements has been a longstanding fun important and what newknowledge can it bring? damental question in nuclear science. How can they survive the Not every combination ofneutrons and protons makes a sta huge electrostatic repulsion? What are their properties? How ble nucleus. Our Earth is home to 81 stable elements, including large is the region of superheavy elements? We do not know yet slightly fewer than 300 stable nuclei. Other nuclei found in all the answers to these questions. This short article presents the nature, although bound to the emission ofprotons and neutrons, current status ofresearch in this field. are radioactive. That is, they eventually capture or emit electrons and positrons, alpha particles, or undergo spontaneous fission. Historical Background Each unstable isotope is characterized by its half-life (T1/2) - the The quest for superheavy nuclei began in the 1940s with the syn time it takes for half of the sample to decay. -

Three Related Topics on the Periodic Tables of Elements
Three related topics on the periodic tables of elements Yoshiteru Maeno*, Kouichi Hagino, and Takehiko Ishiguro Department of physics, Kyoto University, Kyoto 606-8502, Japan * [email protected] (The Foundations of Chemistry: received 30 May 2020; accepted 31 July 2020) Abstaract: A large variety of periodic tables of the chemical elements have been proposed. It was Mendeleev who proposed a periodic table based on the extensive periodic law and predicted a number of unknown elements at that time. The periodic table currently used worldwide is of a long form pioneered by Werner in 1905. As the first topic, we describe the work of Pfeiffer (1920), who refined Werner’s work and rearranged the rare-earth elements in a separate table below the main table for convenience. Today’s widely used periodic table essentially inherits Pfeiffer’s arrangements. Although long-form tables more precisely represent electron orbitals around a nucleus, they lose some of the features of Mendeleev’s short-form table to express similarities of chemical properties of elements when forming compounds. As the second topic, we compare various three-dimensional helical periodic tables that resolve some of the shortcomings of the long-form periodic tables in this respect. In particular, we explain how the 3D periodic table “Elementouch” (Maeno 2001), which combines the s- and p-blocks into one tube, can recover features of Mendeleev’s periodic law. Finally we introduce a topic on the recently proposed nuclear periodic table based on the proton magic numbers (Hagino and Maeno 2020). Here, the nuclear shell structure leads to a new arrangement of the elements with the proton magic-number nuclei treated like noble-gas atoms. -

22.05 Reactor Physics – Part One Course Introduction
22.05 Reactor Physics – Part One Course Introduction 1. Instructor: John A. Bernard 2. Organization: Homework (20%) Four Exams (20% each; lowest grade is dropped) Final Exam (3.0 hours) (20%) 3. Text: The text book for this course is: Introduction to Nuclear Engineering, 3rd Edition, by John Lamarsh. This covers basic reactor physics as part of a complete survey of nuclear engineering. Readings may also be assigned from certain of the books listed below: Nuclear Reactor Analysis by A. F. Henry Introduction to Nuclear Power by G. Hewitt and J. Collier Fundamentals of Nuclear Science and Engineering by J. Shultis and R. Faw Atoms, Radiation, and Radiation Protection by J. Turner Nuclear Criticality Safety by R. Kneif Radiation Detection and Measurement by G. Knoll 4. Course Objective: To quote the late Professor Allan Henry: “The central problem of reactor physics can be stated quite simply. It is to compute, for any time t, the characteristics of the free-neutron population throughout an extended region of space containing an arbitrary, but known, mixture of materials. Specifically we wish to know the number of neutrons in any infinitesimal volume dV that have kinetic energies between E and E + ∆E and are traveling in directions within an infinitesimal angle of a fixed direction specified by the unit vector Ω. If this number is known, we can use the basic data obtained experimentally and theoretically from low-energy neutron physics to predict the rates at which all possible nuclear reactions, including fission, will take place throughout the region. Thus we can 1 predict how much nuclear power will be generated at any given time at any location in the region.” There are several reasons for needing this information: Physical understanding of reactor safety so that both design and operation is done intelligently. -
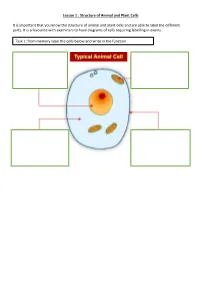
Lesson 1 : Structure of Animal and Plant Cells
Lesson 1 : Structure of Animal and Plant Cells It is important that you know the structure of animal and plant cells and are able to label the different parts. It is a favourite with examiners to have diagrams of cells requiring labelling in exams. Task 1: from memory label the cells below and write in the function Check your answers: There are many similarities and differences between animal and plant cells. Make sure you know these. Similarities Differences 1. Have a nucleus 1. Plant cells have a cellulose cell wall 2. Have a cytoplasm 2. Plant cells have a vacuole containing cell sap 3. Have a cell 3. Plant cells have chloroplast membrane 4. Contain 4. Many plant cells have a box-like shape whilst animal cell shape varies mitochondria 5. Plant cells have the nucleus to the side of the cell, animal cells have a nucleus in 5. Contain ribosomes the middle Task 2: Complete the sentences by filling in the gaps. Both plant and animal cells contain a nucleus. This holds genetic information. Both animal and plant cells have a cell membrane. This controls what enters and leaves the cell. Only a plant cell contains chloroplasts. This is where photosynthesis happens. Both cells contain mitochondria. This is where respiration occurs. Check your answers: Both plant and animal cells contain a nucleus. This holds genetic information. Both animal and plant cells have a cell membrane. This controls what enters and leaves the cell. Only a plant cell contains chloroplasts. This is where photosynthesis happens. Both cells contain mitochondria. This is where respiration occurs. -

Physics of Superheavy Elements Kouichi Hagino
Frontiers in Science II 2013.11.6 Physics of superheavy elements Kouichi Hagino Nuclear Theory Group, Department of Physics, Tohoku University What is nuclear physics? What are superheavy elements? How to create superheavy elements? What are chemical properties of superheavy elements? Introduction: atoms and atomic nuclei What would you see if you magnified the dog? ~ 50 cm Introduction: atoms and atomic nuclei cells ~ 50 cm ~ m = 10-6 m Introduction: atoms and atomic nuclei DNA cells -8 ~ 50 cm ~ m = 10-6 m ~ 10 m atom All things are made of atoms. ~ 10-10 m All things are made of atoms. • Thales, Democritus (ancient Greek) • Dalton (chemist, 19th century) • Boltzmann(19th century) • Einstein (1905) ~ 10-10 m STM image (surface physics group, Tohoku university) Introduction: atoms and atomic nuclei DNA cells -8 ~ 50 cm ~ 10 m atom atomic nucleus ~ 10-15 m ~ 10-10 m proton (+e) neutron (no charge) electron cloud (-e) Neutral atoms: # of protons = # of electrons Chemical properties of atoms # of electrons Mp ~ Mn ~ 2000 Me the mass of atom ~ the mass of nucleus Periodic table of chemical elements tabular arrangement of chemical elements based on the atomic numbers (= # of electrons = # of protons) What are we made of ? oxygen 43 kg cerium 40 mg gallium 0.7 mg carbon 16 kg barium 22 mg tellurium 0.7 mg hydrogen 7 kg iodine 20 mg yttrium 0.6 mg nitrogen 1.8 kg tin 20 mg bismuth 0.5 mg calcium 1.0 kg titanium 20 mg thallium 0.5 mg phosphorus 780 g boron 18 mg indium 0.4 mg potassium 140 g nickel 15 mg gold 0.2 mg sulphur 140 g selenium -

Section 29-4: the Chart of the Nuclides
29-4 The Chart of the Nuclides A nuclide is an atom that is characterized by what is in its nucleus. In other words, it is characterized by the number of protons it has, and by the number of neutrons it has. Figure 29.2 shows the chart of the nuclides, which plots, for stable and radioactive nuclides, the value of Z (the atomic number), on the vertical axis, and the value of N (the number of neutrons) on the horizontal axis. If you look at a horizontal line through the chart, you will find nuclides that all have the same number of protons, but a different number of neutrons. These are all nuclides of the same element, and are known as isotopes (equal proton number, different neutron number). On a vertical line through the chart, the nuclides all have the same number of neutrons, but a different number of protons. These nuclides are known as isotones (equal neutron number, different proton number). It is interesting to think about how the various decay processes play a role in the chart of the nuclides. Only the nuclides shown in black are stable. The stable nuclides give the chart a line of stability that goes from the bottom left toward the upper right, curving down and away from the Z = N line as you move toward higher N values. There are many more nuclides that are shown on the chart but which are not stable - these nuclides decay by means of a radioactive decay process. In general, the dominant decay process for a particular nuclide is the process that produces a nucleus closer to the line of stability than where it started.