Cephalopods of the Broad Caribbean: Distribution, Abundance, and Ecological Importance
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Review of Southern Ocean Squids Using Nets and Beaks
Marine Biodiversity (2020) 50:98 https://doi.org/10.1007/s12526-020-01113-4 REVIEW A review of Southern Ocean squids using nets and beaks Yves Cherel1 Received: 31 May 2020 /Revised: 31 August 2020 /Accepted: 3 September 2020 # Senckenberg Gesellschaft für Naturforschung 2020 Abstract This review presents an innovative approach to investigate the teuthofauna from the Southern Ocean by combining two com- plementary data sets, the literature on cephalopod taxonomy and biogeography, together with predator dietary investigations. Sixty squids were recorded south of the Subtropical Front, including one circumpolar Antarctic (Psychroteuthis glacialis Thiele, 1920), 13 circumpolar Southern Ocean, 20 circumpolar subantarctic, eight regional subantarctic, and 12 occasional subantarctic species. A critical evaluation removed five species from the list, and one species has an unknown taxonomic status. The 42 Southern Ocean squids belong to three large taxonomic units, bathyteuthoids (n = 1 species), myopsids (n =1),andoegopsids (n = 40). A high level of endemism (21 species, 50%, all oegopsids) characterizes the Southern Ocean teuthofauna. Seventeen families of oegopsids are represented, with three dominating families, onychoteuthids (seven species, five endemics), ommastrephids (six species, three endemics), and cranchiids (five species, three endemics). Recent improvements in beak identification and taxonomy allowed making new correspondence between beak and species names, such as Galiteuthis suhmi (Hoyle 1886), Liguriella podophtalma Issel, 1908, and the recently described Taonius notalia Evans, in prep. Gonatus phoebetriae beaks were synonymized with those of Gonatopsis octopedatus Sasaki, 1920, thus increasing significantly the number of records and detailing the circumpolar distribution of this rarely caught Southern Ocean squid. The review extends considerably the number of species, including endemics, recorded from the Southern Ocean, but it also highlights that the corresponding species to two well-described beaks (Moroteuthopsis sp. -
Common Name: Chiton Class: Polyplacophora
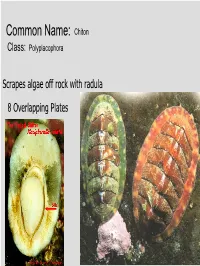
Common Name: Chiton Class: Polyplacophora Scrapes algae off rock with radula 8 Overlapping Plates Phylum? Mollusca Class? Gastropoda Common name? Brown sea hare Class? Scaphopoda Common name? Tooth shell or tusk shell Mud Tentacle Foot Class? Gastropoda Common name? Limpet Phylum? Mollusca Class? Bivalvia Class? Gastropoda Common name? Brown sea hare Phylum? Mollusca Class? Gastropoda Common name? Nudibranch Class? Cephalopoda Cuttlefish Octopus Squid Nautilus Phylum? Mollusca Class? Gastropoda Most Bivalves are Filter Feeders A B E D C • A: Mantle • B: Gill • C: Mantle • D: Foot • E: Posterior adductor muscle I.D. Green: Foot I.D. Red Gills Three Body Regions 1. Head – Foot 2. Visceral Mass 3. Mantle A B C D • A: Radula • B: Mantle • C: Mouth • D: Foot What are these? Snail Radulas Dorsal HingeA Growth line UmboB (Anterior) Ventral ByssalC threads Mussel – View of Outer Shell • A: Hinge • B: Umbo • C: Byssal threads Internal Anatomy of the Bay Mussel A B C D • A: Labial palps • B: Mantle • C: Foot • D: Byssal threads NacreousB layer Posterior adductorC PeriostracumA muscle SiphonD Mantle Byssal threads E Internal Anatomy of the Bay Mussel • A: Periostracum • B: Nacreous layer • C: Posterior adductor muscle • D: Siphon • E: Mantle Byssal gland Mantle Gill Foot Labial palp Mantle Byssal threads Gill Byssal gland Mantle Foot Incurrent siphon Byssal Labial palp threads C D B A E • A: Foot • B: Gills • C: Posterior adductor muscle • D: Excurrent siphon • E: Incurrent siphon Heart G F H E D A B C • A: Foot • B: Gills • C: Mantle • D: Excurrent siphon • E: Incurrent siphon • F: Posterior adductor muscle • G: Labial palps • H: Anterior adductor muscle Siphon or 1. -

Biodiversity and the Future of the Gulf of Maine Area Lewis Incze and Peter Lawton Genes
Biodiversity and the Future of the Gulf of Maine Area Lewis Incze and Peter Lawton Genes Biodiversity is the diversity of life at all levels of organization, from genes to species, communities and ecosystems. Species Nearshore Offshore Bank Basin Slope GoMA: Ecosystem Field Project Habitats and Seamount Communities Abyssal Plain From microbes to whales, and from fundamental biodiversity to EBM GoMA Areas of Work: Species in the Gulf of Maine Area Ecology: past and present Technology Synthesizing Knowledge Linkages to EBM Outreach Today’s Agenda: 08:45-09:45 Presentation: The Global Census and GoMA: What did we do? What did we learn? 09:45-10:00 Q&A 10:00-10:20 BREAK 10:20-11:00 Presentation: Pathways to EBM 11:00-11:45 Discussion Programs of the Census of Marine Life ArCoD Arctic CMarZ Zooplankton CAML Antarctic Creefs Coral Reefs CenSeam Seamounts GoMA Gulf of Maine Area CheSS Chemosynthetic Systems ICOMM Microbes COMARGE Continental margins MAR-ECO Mid-Ocean Ridges CeDAMAR Abyssal Plains NaGISA Intertidal/Shallow Subtidal CenSeam Seamounts TOPP Top Predators HMAP History of Marine Animal Populations FMAP Future of “ “ “ OBIS Ocean Biogeographic Information System Collaborators/Affiliated programs Great Barrier Reef Gulf of Mexico BarCode of Life Encyclopedia of Life Oceans film 10 years (2000-2010) 80 countries, 2700 scientists 17 projects, 14 field projects + OBIS, HMAP Xxx cruises, xxxx days at sea, and FMAP ~ $77m leveraged ~ $767 m --need to 5 affiliated projects (field and technology) check 9 national and regional committees >2,500 scientific papers (many covers) books special journal volumes ~1,200 new species identified >1,500 species in waiting Collection in PLoS-ONE, 2010, incl. -

Genetic Identification of Octopodidae Species in Southern California Seafood Markets: Species Diversity and Resource Implications
Genetic Identification of Octopodidae Species in Southern California Seafood Markets: Species Diversity and Resource Implications Chase Martin Center for Marine Biodiversity and Conservation Scripps Institution of Oceanography University of California San Diego Abstract Various species of Octopodidae are commonly found in seafood markets throughout Southern California. Most of the octopus available for purchase is imported, with the majority of imports coming from various Asian nations. Despite the diversity of global octopus species, products are most commonly labeled as simply “octopus,” with some distinctions being made in size, e.g., “baby” or “little octopus.” In efforts to characterize species diversity, this study genetically tested 59 octopus samples from a variety of seafood markets in Los Angeles, Orange, and San Diego Counties. Universal 16S rRNA primers (ref) and CO1 primers developed by Folmer et al. (1994) were used for PCR amplification and sequencing of mtDNA. In all, 105 sequences were acquired. Seven species were identified with some confidence. Amphioctopus aegina was the most prevalent species, while two additional species were undetermined. Little available data exists pertaining to octopus fisheries of the countries of production of the samples. Most available information on octopus fisheries pertains to those of Mediterranean and North African nations, and identifies the Octopus vulgaris as the fished species. Characterizing octopus diversity in Southern California seafood markets and assessing labeling and countries of production provides the necessary first step for assessing the possible management implications of these fisheries and seafood supply chain logistics for this group of cephalopods. Introduction Octopuses are exclusively marine cephalopod mollusks that form the order Octopoda. -

7. Index of Scientific and Vernacular Names
Cephalopods of the World 249 7. INDEX OF SCIENTIFIC AND VERNACULAR NAMES Explanation of the System Italics : Valid scientific names (double entry by genera and species) Italics : Synonyms, misidentifications and subspecies (double entry by genera and species) ROMAN : Family names ROMAN : Scientific names of divisions, classes, subclasses, orders, suborders and subfamilies Roman : FAO names Roman : Local names 250 FAO Species Catalogue for Fishery Purposes No. 4, Vol. 1 A B Acanthosepion pageorum .....................118 Babbunedda ................................184 Acanthosepion whitleyana ....................128 bandensis, Sepia ..........................72, 138 aculeata, Sepia ............................63–64 bartletti, Blandosepia ........................138 acuminata, Sepia..........................97,137 bartletti, Sepia ............................72,138 adami, Sepia ................................137 bartramii, Ommastrephes .......................18 adhaesa, Solitosepia plangon ..................109 bathyalis, Sepia ..............................138 affinis, Sepia ...............................130 Bathypolypus sponsalis........................191 affinis, Sepiola.......................158–159, 177 Bathyteuthis .................................. 3 African cuttlefish..............................73 baxteri, Blandosepia .........................138 Ajia-kouika .................................. 115 baxteri, Sepia.............................72,138 albatrossae, Euprymna ........................181 belauensis, Nautilus .....................51,53–54 -

Os Nomes Galegos Dos Moluscos
A Chave Os nomes galegos dos moluscos 2017 Citación recomendada / Recommended citation: A Chave (2017): Nomes galegos dos moluscos recomendados pola Chave. http://www.achave.gal/wp-content/uploads/achave_osnomesgalegosdos_moluscos.pdf 1 Notas introdutorias O que contén este documento Neste documento fornécense denominacións para as especies de moluscos galegos (e) ou europeos, e tamén para algunhas das especies exóticas máis coñecidas (xeralmente no ámbito divulgativo, por causa do seu interese científico ou económico, ou por seren moi comúns noutras áreas xeográficas). En total, achéganse nomes galegos para 534 especies de moluscos. A estrutura En primeiro lugar preséntase unha clasificación taxonómica que considera as clases, ordes, superfamilias e familias de moluscos. Aquí apúntase, de maneira xeral, os nomes dos moluscos que hai en cada familia. A seguir vén o corpo do documento, onde se indica, especie por especie, alén do nome científico, os nomes galegos e ingleses de cada molusco (nalgún caso, tamén, o nome xenérico para un grupo deles). Ao final inclúese unha listaxe de referencias bibliográficas que foron utilizadas para a elaboración do presente documento. Nalgunhas desas referencias recolléronse ou propuxéronse nomes galegos para os moluscos, quer xenéricos quer específicos. Outras referencias achegan nomes para os moluscos noutras linguas, que tamén foron tidos en conta. Alén diso, inclúense algunhas fontes básicas a respecto da metodoloxía e dos criterios terminolóxicos empregados. 2 Tratamento terminolóxico De modo moi resumido, traballouse nas seguintes liñas e cos seguintes criterios: En primeiro lugar, aprofundouse no acervo lingüístico galego. A respecto dos nomes dos moluscos, a lingua galega é riquísima e dispomos dunha chea de nomes, tanto específicos (que designan un único animal) como xenéricos (que designan varios animais parecidos). -

Study on the Economic Benefits of Marine Protected Areas Literature Review Analysis
Study on the economic benefits of Marine Protected Areas Literature review analysis Written by ICF Consulting Services Limited, in association with IEEP and PML Sept 2017 EUROPEAN COMMISSION Executive Agency for Small and Medium-sized Enterprises (EASME) Unit A.3 — EMFF E-mail: [email protected] European Commission B-1049 Brussels EUROPEAN COMMISSION Study on the economic benefits of Marine Protected Areas Literature review analysis Executive Agency for Small and Medium-sized Enterprises (EASME) Contract No EASME/EMFF/2015/1.3.1.8/SI2.737373 2018 EN Study on the economic benefits of Marine Protected Areas Europe Direct is a service to help you find answers to your questions about the European Union. Freephone number (*): 00 800 6 7 8 9 10 11 (*) The information given is free, as are most calls (though some operators, phone boxes or hotels may charge you). LEGAL NOTICE This document has been prepared for the European Commission however it reflects the views only of the authors, and the Commission cannot be held responsible for any use which may be made of the information contained therein. More information on the European Union is available on the Internet (http://www.europa.eu). Luxembourg: Publications Office of the European Union, 2018 ISBN 978-92-9202-379-9 DOI 10.2826/40733 © European Union, 2018 Study on the economic benefits of Marine Protected Areas Report authors Pantzar, Mia (IEEP) Russi, Daniela (IEEP) Hooper, Tara (PML) Haines, Rupert (ICF) Quality review Rayment, Matt (ICF) Kettunen, Marianne (IEEP) Study on the economic benefits of Marine Protected Areas Table of Contents Executive summary .......................................................................................... -

Husbandry Manual for BLUE-RINGED OCTOPUS Hapalochlaena Lunulata (Mollusca: Octopodidae)
Husbandry Manual for BLUE-RINGED OCTOPUS Hapalochlaena lunulata (Mollusca: Octopodidae) Date By From Version 2005 Leanne Hayter Ultimo TAFE v 1 T A B L E O F C O N T E N T S 1 PREFACE ................................................................................................................................ 5 2 INTRODUCTION ...................................................................................................................... 6 2.1 CLASSIFICATION .............................................................................................................................. 8 2.2 GENERAL FEATURES ....................................................................................................................... 8 2.3 HISTORY IN CAPTIVITY ..................................................................................................................... 9 2.4 EDUCATION ..................................................................................................................................... 9 2.5 CONSERVATION & RESEARCH ........................................................................................................ 10 3 TAXONOMY ............................................................................................................................12 3.1 NOMENCLATURE ........................................................................................................................... 12 3.2 OTHER SPECIES ........................................................................................................................... -

A Short Note on the Cephalopods Sampled in the Angola Basin During the DIVA-1 Expedition Uwe Piatkowskiã, Rabea Diekmann
ARTICLE IN PRESS Organisms, Diversity & Evolution 5 (2005) 227–230 www.elsevier.de/ode RESULTS OF THE DIVA-1 EXPEDITION OF RV ‘‘METEOR’’ (CRUISE M48/1) A short note on the cephalopods sampled in the Angola Basin during the DIVA-1 expedition Uwe PiatkowskiÃ, Rabea Diekmann IFM-GEOMAR, Leibniz-Institut fu¨r Meereswissenschaften an der Universita¨t Kiel, Du¨sternbrooker Weg 20, D-24105 Kiel, Germany Abstract Five cephalopods, all belonging to different species, were identified from deep-sea trawl samples conducted during the DIVA 1-expedition of RV ‘‘Meteor’’ in the Angola Basin in July 2000. These were the teuthoid squids Bathyteuthis abyssicola, Brachioteuthis riisei, Mastigoteuthis atlantica, Galiteuthis armata, and the finned deep-sea octopus Grimpoteuthis wuelkeri. The present study contributes information on size, morphometry, biology and distribution of the species form this unique cephalopod collection. r 2004 Elsevier GmbH. All rights reserved. Keywords: Cephalopoda; Deep-sea; Angola Basin; Cirrate octopods Introduction captured. These circumstances demonstrate the great scientific value of any cephalopod sampled from deep- Cephalopods in the bathyal and abyssal ecosystems sea habitats. The abyssal plains still belong to the most have been the subject of only a limited number of studies unknown regions in the oceans. One of these plains, the due to the obvious difficulties involved in collecting Angola Basin was sampled during the RV ‘‘Meteor’’ them adequately at such great depths (Voss 1967; expedition in 2000. In the present study, we provide Villanueva 1992). A further drawback relates to their information on a small collection of cephalopods which delicate bodies, which are frequently damaged almost have been caught during the expedition and which beyond recognition in trawl samples. -

Argonauta Argo Linnaeus, 1758
Argonauta argo Linnaeus, 1758 AphiaID: 138803 PAPER NAUTILUS Animalia (Reino) > Mollusca (Filo) > Cephalopoda (Classe) > Coleoidea (Subclasse) > Octopodiformes (Superordem) > Octopoda (Ordem) > Incirrata (Subordem) > Argonautoidea (Superfamilia) > Argonautidae (Familia) Natural History Museum Rotterdam Natural History Museum Rotterdam Sinónimos Argonauta argo f. agglutinans Martens, 1867 Argonauta argo f. aurita Martens, 1867 Argonauta argo f. mediterranea Monterosato, 1914 Argonauta argo f. obtusangula Martens, 1867 Argonauta argo var. americana Dall, 1889 Argonauta bulleri Kirk, 1886 Argonauta compressus Blainville, 1826 Argonauta corrugata Humphrey, 1797 Argonauta corrugatus Humphrey, 1797 Argonauta cygnus Monterosato, 1889 Argonauta dispar Conrad, 1854 Argonauta ferussaci Monterosato, 1914 Argonauta grandiformis Perry, 1811 Argonauta haustrum Dillwyn, 1817 Argonauta haustrum Wood, 1811 Argonauta minor Risso, 1854 Argonauta monterosatoi Monterosato, 1914 Argonauta monterosatoi Coen, 1915 Argonauta naviformis Conrad, 1854 1 Argonauta pacificus Dall, 1871 Argonauta papyraceus Röding, 1798 Argonauta papyria Conrad, 1854 Argonauta papyrius Conrad, 1854 Argonauta sebae Monterosato, 1914 Argonauta sulcatus Lamarck, 1801 Ocythoe antiquorum Leach, 1817 Ocythoe argonautae (Cuvier, 1829) Referências original description Linnaeus, C. (1758). Systema Naturae per regna tria naturae, secundum classes, ordines, genera, species, cum characteribus, differentiis, synonymis, locis. Editio decima, reformata. Laurentius Salvius: Holmiae. ii, 824 pp., available -

555 Nautilus Bercangkang Rapuh Dari Teluk Tomini
Open Access, August 2020 J. Ilmu dan Teknologi Kelautan Tropis, 12(2): 555-563 p-ISSN : 2087-9423 http://journal.ipb.ac.id/index.php/jurnalikt e-ISSN : 2620-309X DOI: http://doi.org/10.29244/jitkt.v12i2.25795 NAUTILUS BERCANGKANG RAPUH DARI TELUK TOMINI KABUPATEN PARIGI MOUTONG SULAWESI TENGAH, INDONESIA PAPER NAUTILUSES FROM TOMINI BAY PARIGI MOUTONG REGENCY CENTRAL SULAWESI, INDONESIA Fina Saffuteri Sarif1*, Delianis Pringgenies2, Agus Hartoko2, & Mada T Sibero2 1Program Studi Manajemen Sumberdaya Pantai, Fakultas Perikanan dan Ilmu Kelautan, Universitas Diponegoro, Semarang, 50275, Indonesia 2Departemen Ilmu Kelautan, FPIK, Universitas Diponegoro, Semarang, 50275, Indonesia *E-mail: [email protected] ABSTRACT Paper nautiluses are classified as Cephalopoda class, Argonautidae family. The aims of this research to identification of shell morphological characters of paper Nautiluses were collected at 1,000 m depth. The results showed that out of all the samples successfully collected during the course of the study (March till December 2016), only 6 specimens were found at 70-80 depth, with 4 of those species are egg-laying, and the other 2 are not. From the 6 species found, 2 were Argonauta argo with average shell length of 34.05 mm, shell width of 22.20 mm, and average aperture width of 11.15 mm. A. argo is known to possess 8 tentacles, 4 long and 2 short appendages. The shell color is brighter than that of A. hians, with flatter shell and two strips of keels located near each other along the dorsal and soft side, along ventral side thickens and sharpens. A. hians possess average shell length of 47.02 mm, shell width of 33.07 mm and aperture width of 21.30 mm. -

Octopus Insularis</Italic> As a New Marine Model for Evolutionary
© 2019. Published by The Company of Biologists Ltd | Biology Open (2019) 8, bio046086. doi:10.1242/bio.046086 RESEARCH ARTICLE Octopus insularis as a new marine model for evolutionary developmental biology Ernesto Maldonado1,*, Emma Rangel-Huerta1,2, Roberto González-Gómez3,4, Gabriel Fajardo-Alvarado3,4 and Piedad S. Morillo-Velarde4,5,* ABSTRACT of aquatic animal eggs and embryos guarantees the observation of Octopuses are intriguing organisms that, together with squids and every developmental stage using microscopy and allows detailed cuttlefishes, form the extant coleoid cephalopods. This group includes experimental analysis from the first cell division through to the many species that can potentially be used as models in the fields of formation of embryonic germ layers and organogenesis (Boletzky biomedicine, developmental biology, evolution, neuroscience and et al., 2006). Finally, small embryos allow reasonable sample sizes even for robotics research. The purpose of this work is to first to be tested together using multi-well plates to provide multiple present a simple method for maintaining Octopus insularis embryos experimental replicates at the same time, making them cost- under a laboratory setup. Second, we show that these embryos are effective animal models (Hill et al., 2005). suitable for detailed analyses of specific traits that appear during Coleoid cephalopods (octopus, squid and cuttlefish) exhibit the developmental stages, including the eyes, hearts, arms, suckers, largest nervous systems found among invertebrates (Young, 1971) chromatophores and Kölliker’s organs. Similar complex traits between and a sophisticated visual system controlling body color changes for cephalopods and vertebrates such as the visual, cardiovascular, communication, camouflage and mimicry (Hanlon et al., 2011; neural and pigmentation systems are generally considered to be a Robin et al., 2014).