An Equation of State for Metals at High Temperature and Pressure in Compressed and Expanded Volume Regions
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

LATENT HEAT of FUSION INTRODUCTION When a Solid Has Reached Its Melting Point, Additional Heating Melts the Solid Without a Temperature Change
LATENT HEAT OF FUSION INTRODUCTION When a solid has reached its melting point, additional heating melts the solid without a temperature change. The temperature will remain constant at the melting point until ALL of the solid has melted. The amount of heat needed to melt the solid depends upon both the amount and type of matter that is being melted. Therefore: Q = ML Eq. 1 f where Q is the amount of heat absorbed by the solid, M is the mass of the solid that was melted and Lf is the latent heat of fusion for the type of material that was melted, which is measured in J/kg, NOTE: to fuse means to melt. In this experiment the latent heat of fusion of water will be determined by using the method of mixtures ΣQ=0, or QGained +QLost =0. Ice will be added to a calorimeter containing warm water. The heat energy lost by the water and calorimeter does two things: 1. It melts the ice; 2. It warms the water formed by the melting ice from zero to the final equilibrium temperature of the mixture. Heat gained + Heat lost = 0 Heat needed to melt ice + Heat needed to warm the melt water + Heat lost by warn water = 0 MiceLf + MiceCw (Tf - 0) + MwCw (Tf – Tw) = 0 Eq. 2. * Note: The mass of the melted water is the same as the mass of the ice. where M = mass of warm water initially in calorimeter w Mice = mass of ice and water from the melted ice Cw = specific heat of water Lf = latent heat of fusion of water Tw = initial temperature water Tf = equilibrium temperature of mixture APPARATUS: Calorimeter, thermometer, balance, ice, water OBJECTIVE: To determine the latent heat of fusion of water PROCEDURE: 1. -

Thermodynamics Notes
Thermodynamics Notes Steven K. Krueger Department of Atmospheric Sciences, University of Utah August 2020 Contents 1 Introduction 1 1.1 What is thermodynamics? . .1 1.2 The atmosphere . .1 2 The Equation of State 1 2.1 State variables . .1 2.2 Charles' Law and absolute temperature . .2 2.3 Boyle's Law . .3 2.4 Equation of state of an ideal gas . .3 2.5 Mixtures of gases . .4 2.6 Ideal gas law: molecular viewpoint . .6 3 Conservation of Energy 8 3.1 Conservation of energy in mechanics . .8 3.2 Conservation of energy: A system of point masses . .8 3.3 Kinetic energy exchange in molecular collisions . .9 3.4 Working and Heating . .9 4 The Principles of Thermodynamics 11 4.1 Conservation of energy and the first law of thermodynamics . 11 4.1.1 Conservation of energy . 11 4.1.2 The first law of thermodynamics . 11 4.1.3 Work . 12 4.1.4 Energy transferred by heating . 13 4.2 Quantity of energy transferred by heating . 14 4.3 The first law of thermodynamics for an ideal gas . 15 4.4 Applications of the first law . 16 4.4.1 Isothermal process . 16 4.4.2 Isobaric process . 17 4.4.3 Isosteric process . 18 4.5 Adiabatic processes . 18 5 The Thermodynamics of Water Vapor and Moist Air 21 5.1 Thermal properties of water substance . 21 5.2 Equation of state of moist air . 21 5.3 Mixing ratio . 22 5.4 Moisture variables . 22 5.5 Changes of phase and latent heats . -

Equations of State and Thermodynamics of Solids Using Empirical Corrections in the Quasiharmonic Approximation
PHYSICAL REVIEW B 84, 184103 (2011) Equations of state and thermodynamics of solids using empirical corrections in the quasiharmonic approximation A. Otero-de-la-Roza* and V´ıctor Luana˜ † Departamento de Qu´ımica F´ısica y Anal´ıtica, Facultad de Qu´ımica, Universidad de Oviedo, ES-33006 Oviedo, Spain (Received 24 May 2011; revised manuscript received 8 October 2011; published 11 November 2011) Current state-of-the-art thermodynamic calculations using approximate density functionals in the quasi- harmonic approximation (QHA) suffer from systematic errors in the prediction of the equation of state and thermodynamic properties of a solid. In this paper, we propose three simple and theoretically sound empirical corrections to the static energy that use one, or at most two, easily accessible experimental parameters: the room-temperature volume and bulk modulus. Coupled with an appropriate numerical fitting technique, we show that experimental results for three model systems (MgO, fcc Al, and diamond) can be reproduced to a very high accuracy in wide ranges of pressure and temperature. In the best available combination of functional and empirical correction, the predictive power of the DFT + QHA approach is restored. The calculation of the volume-dependent phonon density of states required by QHA can be too expensive, and we have explored simplified thermal models in several phases of Fe. The empirical correction works as expected, but the approximate nature of the simplified thermal model limits significantly the range of validity of the results. DOI: 10.1103/PhysRevB.84.184103 PACS number(s): 64.10.+h, 65.40.−b, 63.20.−e, 71.15.Nc I. -

Determination of the Identity of an Unknown Liquid Group # My Name the Date My Period Partner #1 Name Partner #2 Name
Determination of the Identity of an unknown liquid Group # My Name The date My period Partner #1 name Partner #2 name Purpose: The purpose of this lab is to determine the identity of an unknown liquid by measuring its density, melting point, boiling point, and solubility in both water and alcohol, and then comparing the results to the values for known substances. Procedure: 1) Density determination Obtain a 10mL sample of the unknown liquid using a graduated cylinder Determine the mass of the 10mL sample Save the sample for further use 2) Melting point determination Set up an ice bath using a 600mL beaker Obtain a ~5mL sample of the unknown liquid in a clean dry test tube Place a thermometer in the test tube with the sample Place the test tube in the ice water bath Watch for signs of crystallization, noting the temperature of the sample when it occurs Save the sample for further use 3) Boiling point determination Set up a hot water bath using a 250mL beaker Begin heating the water in the beaker Obtain a ~10mL sample of the unknown in a clean, dry test tube Add a boiling stone to the test tube with the unknown Open the computer interface software, using a graph and digit display Place the temperature sensor in the test tube so it is in the unknown liquid Record the temperature of the sample in the test tube using the computer interface Watch for signs of boiling, noting the temperature of the unknown Dispose of the sample in the assigned waste container 4) Solubility determination Obtain two small (~1mL) samples of the unknown in two small test tubes Add an equal amount of deionized into one of the samples Add an equal amount of ethanol into the other Mix both samples thoroughly Compare the samples for solubility Dispose of the samples in the assigned waste container Observations: The unknown is a clear, colorless liquid. -
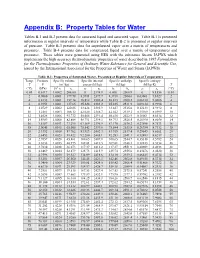
Appendix B: Property Tables for Water
Appendix B: Property Tables for Water Tables B-1 and B-2 present data for saturated liquid and saturated vapor. Table B-1 is presented information at regular intervals of temperature while Table B-2 is presented at regular intervals of pressure. Table B-3 presents data for superheated vapor over a matrix of temperatures and pressures. Table B-4 presents data for compressed liquid over a matrix of temperatures and pressures. These tables were generated using EES with the substance Steam_IAPWS which implements the high accuracy thermodynamic properties of water described in 1995 Formulation for the Thermodynamic Properties of Ordinary Water Substance for General and Scientific Use, issued by the International Associated for the Properties of Water and Steam (IAPWS). Table B-1: Properties of Saturated Water, Presented at Regular Intervals of Temperature Temp. Pressure Specific volume Specific internal Specific enthalpy Specific entropy T P (m3/kg) energy (kJ/kg) (kJ/kg) (kJ/kg-K) T 3 (°C) (kPa) 10 vf vg uf ug hf hg sf sg (°C) 0.01 0.6117 1.0002 206.00 0 2374.9 0.000 2500.9 0 9.1556 0.01 2 0.7060 1.0001 179.78 8.3911 2377.7 8.3918 2504.6 0.03061 9.1027 2 4 0.8135 1.0001 157.14 16.812 2380.4 16.813 2508.2 0.06110 9.0506 4 6 0.9353 1.0001 137.65 25.224 2383.2 25.225 2511.9 0.09134 8.9994 6 8 1.0729 1.0002 120.85 33.626 2385.9 33.627 2515.6 0.12133 8.9492 8 10 1.2281 1.0003 106.32 42.020 2388.7 42.022 2519.2 0.15109 8.8999 10 12 1.4028 1.0006 93.732 50.408 2391.4 50.410 2522.9 0.18061 8.8514 12 14 1.5989 1.0008 82.804 58.791 2394.1 58.793 2526.5 -

Equation of State of an Ideal Gas
ASME231 Atmospheric Thermodynamics NC A&T State U Department of Physics Dr. Yuh-Lang Lin http://mesolab.org [email protected] Lecture 3: Equation of State of an Ideal Gas As mentioned earlier, the state of a system is the condition of the system (or part of the system) at an instant of time measured by its properties. Those properties are called state variables, such as T, p, and V. The equation which relates T, p, and V is called equation of state. Since they are related by the equation of state, only two of them are independent. Thus, all the other thermodynamic properties will depend on the state defined by two independent variables by state functions. PVT system: The simplest thermodynamic system consists of a fixed mass of a fluid uninfluenced by chemical reactions or external fields. Such a system can be described by the pressure, volume and temperature, which are related by an equation of state f (p, V, T) = 0, (2.1) 1 where only two of them are independent. From physical experiments, the equation of state for an ideal gas, which is defined as a hypothetical gas whose molecules occupy negligible space and have no interactions, may be written as p = RT, (2.2) or p = RT, (2.3) where -3 : density (=m/V) in kg m , 3 -1 : specific volume (=V/m=1/) in m kg . R: specific gas constant (R=R*/M), -1 -1 -1 -1 [R=287 J kg K for dry air (Rd), 461 J kg K for water vapor for water vapor (Rv)]. -

States of Matter Lesson
National Aeronautics and Space Administration STATES OF MATTER NASA SUMMER OF INNOVATION LESSON DESCRIPTION UNIT This lesson explores the states of matter Physical Science—States of Matter and their properties. GRADE LEVELS OBJECTIVES 4 – 6 Students will CONNECTION TO CURRICULUM • Simulate the movement of atoms and molecules in solids, liquids, Science and gases TEACHER PREPARATION TIME • Demonstrate the properties of 2 hours liquids including density and buoyancy LESSON TIME NEEDED • Investigate how the density of a 4 hours Complexity: Moderate solid behaves in varying densities of liquids • Construct a rocket powered by pressurized gas created from a chemical reaction between a solid and a liquid NATIONAL STANDARDS National Science Education Standards (NSTA) Science and Technology • Abilities of technological design • Understanding science and technology Physical Science • Position and movement of objects • Properties and changes in properties of matter • Transfer of energy MANAGEMENT For the first activity you may need to enhance prior knowledge about matter and energy from a supplemental handout called “Diagramming Atoms and Molecules in Motion.” At the middle school level, this information about the invisible world of the atom is often presented as a story which we ask them to accept without much ready evidence. Since so many middle school students have not had science experience at the concrete operational level, they are poorly equipped to work at an abstract level. However, in this activity students can begin to see evidence that supports the abstract information you are sharing with them. They can take notes on the first two descriptions as you present on the overhead. Emphasize the spacing of the particles, rather than the number. -

Specific Energy Limit and Its Influence on the Nature of Black Holes Javier Viaña
Specific Energy Limit and its Influence on the Nature of Black Holes Javier Viaña To cite this version: Javier Viaña. Specific Energy Limit and its Influence on the Nature of Black Holes. 2021. hal- 03322333 HAL Id: hal-03322333 https://hal.archives-ouvertes.fr/hal-03322333 Preprint submitted on 19 Aug 2021 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. 16th of August of 2021 Specific Energy Limit and its Influence on the Nature of Black Holes Javier Viaña [0000-0002-0563-784X] University of Cincinnati, Cincinnati OH 45219, USA [email protected] What if the universe has a limit on the amount of energy that a certain mass can have? This article explores this possibility and suggests a theory for the creation and nature of black holes based on an energetic limit. The Specific Energy Limit Energy is an extensive property, and we know that as we add more mass to a given system, we can easily increase its energy. Specific energy on the other hand is an intensive property. It is defined as the energy divided by the mass and it is measured in units of J/kg. -

Single Crystal Growth of Mgb2 by Using Low Melting Point Alloy Flux
Single Crystal Growth of Magnesium Diboride by Using Low Melting Point Alloy Flux Wei Du*, Xugang Xian, Xiangjin Zhao, Li Liu, Zhuo Wang, Rengen Xu School of Environment and Material Engineering, Yantai University, Yantai 264005, P. R. China Abstract Magnesium diboride crystals were grown with low melting alloy flux. The sample with superconductivity at 38.3 K was prepared in the zinc-magnesium flux and another sample with superconductivity at 34.11 K was prepared in the cadmium-magnesium flux. MgB4 was found in both samples, and MgB4’s magnetization curve being above zero line had not superconductivity. Magnesium diboride prepared by these two methods is all flaky and irregular shape and low quality of single crystal. However, these two metals should be the optional flux for magnesium diboride crystal growth. The study of single crystal growth is very helpful for the future applications. Keywords: Crystal morphology; Low melting point alloy flux; Magnesium diboride; Superconducting materials Corresponding author. Tel: +86-13235351100 Fax: +86-535-6706038 Email-address: [email protected] (W. Du) 1. Introduction Since the discovery of superconductivity in Magnesium diboride (MgB2) with a transition temperature (Tc) of 39K [1], a great concern has been given to this material structure, just as high-temperature copper oxide can be doped by elements to improve its superconducting temperature. Up to now, it is failure to improve Tc of doped MgB2 through C [2-3], Al [4], Li [5], Ir [6], Ag [7], Ti [8], Ti, Zr and Hf [9], and Be [10] on the contrary, the Tc is reduced or even disappeared. -

Thermodynamics
ME346A Introduction to Statistical Mechanics { Wei Cai { Stanford University { Win 2011 Handout 6. Thermodynamics January 26, 2011 Contents 1 Laws of thermodynamics 2 1.1 The zeroth law . .3 1.2 The first law . .4 1.3 The second law . .5 1.3.1 Efficiency of Carnot engine . .5 1.3.2 Alternative statements of the second law . .7 1.4 The third law . .8 2 Mathematics of thermodynamics 9 2.1 Equation of state . .9 2.2 Gibbs-Duhem relation . 11 2.2.1 Homogeneous function . 11 2.2.2 Virial theorem / Euler theorem . 12 2.3 Maxwell relations . 13 2.4 Legendre transform . 15 2.5 Thermodynamic potentials . 16 3 Worked examples 21 3.1 Thermodynamic potentials and Maxwell's relation . 21 3.2 Properties of ideal gas . 24 3.3 Gas expansion . 28 4 Irreversible processes 32 4.1 Entropy and irreversibility . 32 4.2 Variational statement of second law . 32 1 In the 1st lecture, we will discuss the concepts of thermodynamics, namely its 4 laws. The most important concepts are the second law and the notion of Entropy. (reading assignment: Reif x 3.10, 3.11) In the 2nd lecture, We will discuss the mathematics of thermodynamics, i.e. the machinery to make quantitative predictions. We will deal with partial derivatives and Legendre transforms. (reading assignment: Reif x 4.1-4.7, 5.1-5.12) 1 Laws of thermodynamics Thermodynamics is a branch of science connected with the nature of heat and its conver- sion to mechanical, electrical and chemical energy. (The Webster pocket dictionary defines, Thermodynamics: physics of heat.) Historically, it grew out of efforts to construct more efficient heat engines | devices for ex- tracting useful work from expanding hot gases (http://www.answers.com/thermodynamics). -

7 Apr 2021 Thermodynamic Response Functions . L03–1 Review Of
7 apr 2021 thermodynamic response functions . L03{1 Review of Thermodynamics. 3: Second-Order Quantities and Relationships Maxwell Relations • Idea: Each thermodynamic potential gives rise to several identities among its second derivatives, known as Maxwell relations, which express the integrability of the fundamental identity of thermodynamics for that potential, or equivalently the fact that the potential really is a thermodynamical state function. • Example: From the fundamental identity of thermodynamics written in terms of the Helmholtz free energy, dF = −S dT − p dV + :::, the fact that dF really is the differential of a state function implies that @ @F @ @F @S @p = ; or = : @V @T @T @V @V T;N @T V;N • Other Maxwell relations: From the same identity, if F = F (T; V; N) we get two more relations. Other potentials and/or pairs of variables can be used to obtain additional relations. For example, from the Gibbs free energy and the identity dG = −S dT + V dp + µ dN, we get three relations, including @ @G @ @G @S @V = ; or = − : @p @T @T @p @p T;N @T p;N • Applications: Some measurable quantities, response functions such as heat capacities and compressibilities, are second-order thermodynamical quantities (i.e., their definitions contain derivatives up to second order of thermodynamic potentials), and the Maxwell relations provide useful equations among them. Heat Capacities • Definitions: A heat capacity is a response function expressing how much a system's temperature changes when heat is transferred to it, or equivalently how much δQ is needed to obtain a given dT . The general definition is C = δQ=dT , where for any reversible transformation δQ = T dS, but the value of this quantity depends on the details of the transformation. -

Sounds of a Supersolid A
NEWS & VIEWS RESEARCH hypothesis came from extensive population humans, implying possible mosquito exposure long-distance spread of insecticide-resistant time-series analysis from that earlier study5, to malaria parasites and the potential to spread mosquitoes, worsening an already dire situ- which showed beyond reasonable doubt that infection over great distances. ation, given the current spread of insecticide a mosquito vector species called Anopheles However, the authors failed to detect resistance in mosquito populations. This would coluzzii persists locally in the dry season in parasite infections in their aerially sampled be a matter of great concern because insecticides as-yet-undiscovered places. However, the malaria vectors, a result that they assert is to be are the best means of malaria control currently data were not consistent with this outcome for expected given the small sample size and the low available8. However, long-distance migration other malaria vectors in the study area — the parasite-infection rates typical of populations of could facilitate the desirable spread of mosqui- species Anopheles gambiae and Anopheles ara- malaria vectors. A problem with this argument toes for gene-based methods of malaria-vector biensis — leaving wind-powered long-distance is that the typical infection rates they mention control. One thing is certain, Huestis and col- migration as the only remaining possibility to are based on one specific mosquito body part leagues have permanently transformed our explain the data5. (salivary glands), rather than the unknown but understanding of African malaria vectors and Both modelling6 and genetic studies7 undoubtedly much higher infection rates that what it will take to conquer malaria.