M M Ademecu Emecu
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Gross Anatomy
www.BookOfLinks.com THE BIG PICTURE GROSS ANATOMY www.BookOfLinks.com Notice Medicine is an ever-changing science. As new research and clinical experience broaden our knowledge, changes in treatment and drug therapy are required. The authors and the publisher of this work have checked with sources believed to be reliable in their efforts to provide information that is complete and generally in accord with the standards accepted at the time of publication. However, in view of the possibility of human error or changes in medical sciences, neither the authors nor the publisher nor any other party who has been involved in the preparation or publication of this work warrants that the information contained herein is in every respect accurate or complete, and they disclaim all responsibility for any errors or omissions or for the results obtained from use of the information contained in this work. Readers are encouraged to confirm the infor- mation contained herein with other sources. For example and in particular, readers are advised to check the product information sheet included in the package of each drug they plan to administer to be certain that the information contained in this work is accurate and that changes have not been made in the recommended dose or in the contraindications for administration. This recommendation is of particular importance in connection with new or infrequently used drugs. www.BookOfLinks.com THE BIG PICTURE GROSS ANATOMY David A. Morton, PhD Associate Professor Anatomy Director Department of Neurobiology and Anatomy University of Utah School of Medicine Salt Lake City, Utah K. Bo Foreman, PhD, PT Assistant Professor Anatomy Director University of Utah College of Health Salt Lake City, Utah Kurt H. -

Normal Gross and Histologic Features of the Gastrointestinal Tract
NORMAL GROSS AND HISTOLOGIC 1 FEATURES OF THE GASTROINTESTINAL TRACT THE NORMAL ESOPHAGUS left gastric, left phrenic, and left hepatic accessory arteries. Veins in the proximal and mid esopha- Anatomy gus drain into the systemic circulation, whereas Gross Anatomy. The adult esophagus is a the short gastric and left gastric veins of the muscular tube measuring approximately 25 cm portal system drain the distal esophagus. Linear and extending from the lower border of the cri- arrays of large caliber veins are unique to the distal coid cartilage to the gastroesophageal junction. esophagus and can be a helpful clue to the site of It lies posterior to the trachea and left atrium a biopsy when extensive cardiac-type mucosa is in the mediastinum but deviates slightly to the present near the gastroesophageal junction (4). left before descending to the diaphragm, where Lymphatic vessels are present in all layers of the it traverses the hiatus and enters the abdomen. esophagus. They drain to paratracheal and deep The subdiaphragmatic esophagus lies against cervical lymph nodes in the cervical esophagus, the posterior surface of the left hepatic lobe (1). bronchial and posterior mediastinal lymph nodes The International Classification of Diseases in the thoracic esophagus, and left gastric lymph and the American Joint Commission on Cancer nodes in the abdominal esophagus. divide the esophagus into upper, middle, and lower thirds, whereas endoscopists measure distance to points in the esophagus relative to the incisors (2). The esophagus begins 15 cm from the incisors and extends 40 cm from the incisors in the average adult (3). The upper and lower esophageal sphincters represent areas of increased resting tone but lack anatomic landmarks; they are located 15 to 18 cm from the incisors and slightly proximal to the gastroesophageal junction, respectively. -
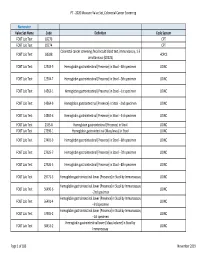
2020 Measure Value Set Colorectal Cancer Screening
PT ‐ 2020 Measure Value Set_Colorectal Cancer Screening Numerator Value Set Name Code Definition Code System FOBT Lab Test 82270 CPT FOBT Lab Test 82274 CPT Colorectal cancer screening; fecal occult blood test, immunoassay, 1‐3 FOBT Lab Test G0328 HCPCS simultaneous (G0328) FOBT Lab Test 12503‐9 Hemoglobin.gastrointestinal [Presence] in Stool ‐‐4th specimen LOINC FOBT Lab Test 12504‐7 Hemoglobin.gastrointestinal [Presence] in Stool ‐‐5th specimen LOINC FOBT Lab Test 14563‐1 Hemoglobin.gastrointestinal [Presence] in Stool ‐‐1st specimen LOINC FOBT Lab Test 14564‐9 Hemoglobin.gastrointestinal [Presence] in Stool ‐‐2nd specimen LOINC FOBT Lab Test 14565‐6 Hemoglobin.gastrointestinal [Presence] in Stool ‐‐3rd specimen LOINC FOBT Lab Test 2335‐8 Hemoglobin.gastrointestinal [Presence] in Stool LOINC FOBT Lab Test 27396‐1 Hemoglobin.gastrointestinal [Mass/mass] in Stool LOINC FOBT Lab Test 27401‐9 Hemoglobin.gastrointestinal [Presence] in Stool ‐‐6th specimen LOINC FOBT Lab Test 27925‐7 Hemoglobin.gastrointestinal [Presence] in Stool ‐‐7th specimen LOINC FOBT Lab Test 27926‐5 Hemoglobin.gastrointestinal [Presence] in Stool ‐‐8th specimen LOINC FOBT Lab Test 29771‐3 Hemoglobin.gastrointestinal.lower [Presence] in Stool by Immunoassay LOINC Hemoglobin.gastrointestinal.lower [Presence] in Stool by Immunoassay FOBT Lab Test 56490‐6 LOINC ‐‐2nd specimen Hemoglobin.gastrointestinal.lower [Presence] in Stool by Immunoassay FOBT Lab Test 56491‐4 LOINC ‐‐3rd specimen Hemoglobin.gastrointestinal.lower [Presence] in Stool by Immunoassay FOBT Lab Test 57905‐2 -

Thieme: Lymphedema Management
8 1 Anatomy tween a proximal and a distal pair of valves is called lymph angion (Fig. 1−4). The media in valvular areas of lymph collectors contains less smooth musculature than the angion area. Lymph angions have an autonomic contraction frequency of ෂ10 to 12 contractions per minute at rest (lymphangiomotoricity). In healthy lymph collectors, the proximal valve is open during the systole, whereas the distal valve is closed; in the diastole, the op- posite is the case. This permits directional flow of lymph fluid from distal to proximal angions. In lymphangiectasia (dilation) with valvular insufficiency, the lymph flow may reverse into distal lymph angions (lymphatic reflux). Lymph collectors have the ability to react to an increase in lymph formation with an in- crease in contraction frequency. The increase in lymph fluid entering the lymph angion will cause a stretch on the wall of the angion, which Figure 1−4 Lymph collectors. 1. Lymph collector; 2. Afferent lymph collector to lymph node; 3. Efferent in turn results in an increase in lymphangio- lymph collector from lymph node; 4. Lymph node; 5. motoricity (lymphatic safety factor; see also Cross section through a lymph collector in the area of Chapter 2, Safety Factor of the Lymphatic Sys- the valves; 6. Lymph angion. tem). Other factors that may influence lymphan- giomotoricity are external stretch on the It is postulated that the main purpose of pre- lymph angion wall (e.g., manual lymph collectors is the transport of lymph fluid from drainage), temperature, activity of muscle and the capillaries to lymph collectors. Due to the joint pumps, diaphragmatic breathing, pulsa- capillary-like wall structure in some areas, pre- tion of adjacent arteries, and certain tissue collectors are able to absorb lymphatic loads. -

Magnetic Resonance Imaging and Endorectal Ultrasound for Diagnosis
Burdan et al. European Journal of Medical Research (2015) 20:4 DOI 10.1186/s40001-014-0078-0 EUROPEAN JOURNAL OF MEDICAL RESEARCH REVIEW Open Access Magnetic resonance imaging and endorectal ultrasound for diagnosis of rectal lesions Franciszek Burdan1,2*, Iwona Sudol-Szopinska3,4, Elzbieta Staroslawska1, Malgorzata Kolodziejczak5, Robert Klepacz6, Agnieszka Mocarska1, Marek Caban1, Iwonna Zelazowska-Cieslinska1 and Justyna Szumilo6 Abstract Endorectal ultrasonography (ERUS) and magnetic resonance imaging (MRI) allow exploring the morphology of the rectum in detail. Use of such data, especially assessment of the rectal wall, is an important tool for ascertaining the perianal fistula localization as well as stage of the cancer and planning it appropriate treatment, as stage T3 tumors are usually treated with neoadjuvant therapy, whereas T2 tumors are initially managed surgically. The only advantage of ERUS over MRI is the possibility of assessing T1 tumors that could be treated by transanal endoscopic microsurgery. However, MRI is better for visualizing most radiological prognostic features in rectal or anal cancer such as a circumferential resection margin less than 1 mm, T stage at T1-T2 or T3 tumors with extramural extension less than 5 mm, absence of extramural vascular invasion, N stage at N0/N1, and tumors located in the middle or upper third of the rectum. It can also evaluate the intersphincteric space or levator ani muscle involvement. Increased signal on diffusion weighted imaging (DWI) and low apparent diffusion coefficient (ADC) values as well as an irregular contour and heterogeneous internal signal intensity seem to predict the involvement of pelvic lymphatic nodes better than their size alone. -

Evidence Tables
Evidence Tables Citation: Akin O, Nessar G, Agildere AM, Aydog G (2004) Preoperative staging of rectal cancer with endorectal MR imaging: Comparison with histopathologic findings. Journal of Clinical Imaging 28;432-438. Design: Prospective Case Series Country: Turkey Aim: to assess the accuracy of endorectal MR imaging in the preoperative local staging of rectal cancers. Inclusion criteria None given Exclusion criteria One patient was excluded due to the endorectal coil not being placed appropriately. One patient was excluded because the neoplasm extended beyond the scope of the endorectal coil One patient was excluded because they refused surgery. Population N=20 Interventions Endorectal MRI Outcomes Sensitivity Specificity Results Endorectal MRI agreed with histopathological staging in 17/20 patients. The overall accuracy of endorectal MRI for determining T stage of rectal tumours was 85%. Histopathologic Staging T1 T2 T3 T4 Total T1 2 1 - - 3 T2 - 2 1 - 3 Endorectal MRI T3 - 1 11 - 12 staging T4 - - - 2 2 Total 2 4 12 2 20 Table: Comparison of staging based on endorectal MRI and histopathologic staging 2 tumours were identified as T1 histopathologically and on endorectal MRI 4 tumours were histopathologically identified as being T2, with 2 of these identified on endorectal MRI. In one case invasion of the muscularis propria was not detected on endorectal MRI resulting in the tumour being under- staged to T1 and in the second case the tumour was over-staged to T3 due to inflammatory changes mimicking tumour invasion into the perirectal fat. 12 tumours were histopathologically identified as T3, 11 of these were correctly identified as T3 by endorectal MRI which clearly demonstrated tumoural invasion of the perirectal fat. -

Definition and Delineation of the Clinical Target Volume for Rectal Cancer Sarah Roels, M.D.,* Wim Duthoy, M.D.,§ Karin Hauster
Int. J. Radiation Oncology Biol. Phys., Vol. 65, No. 4, pp. 1129–1142, 2006 Copyright © 2006 Elsevier Inc. Printed in the USA. All rights reserved 0360-3016/06/$–see front matter doi:10.1016/j.ijrobp.2006.02.050 CLINICAL INVESTIGATION Rectum DEFINITION AND DELINEATION OF THE CLINICAL TARGET VOLUME FOR RECTAL CANCER § SARAH ROELS, M.D.,* WIM DUTHOY, M.D., KARIN HAUSTERMANS, M.D., PH.D.,* † ‡ § FREDDY PENNINCKX, M.D., PH.D., VINCENT VANDECAVEYE, M.D., TOM BOTERBERG, M.D., § AND WILFRIED DE NEVE, M.D., PH.D. Departments of *Radiotherapy, †Surgery, and ‡Radiology, University Hospital Gasthuisberg, Leuven, Belgium; and §Department of Radiotherapy, Ghent University Hospital, Ghent, Belgium Purpose: Optimization of radiation techniques to maximize local tumor control and to minimize small bowel toxicity in locally advanced rectal cancer requires proper definition and delineation guidelines for the clinical target volume (CTV). The purpose of this investigation was to analyze reported data on the predominant locations and frequency of local recurrences and lymph node involvement in rectal cancer, to propose a definition of the CTV for rectal cancer and guidelines for its delineation. Methods and Materials: Seven reports were analyzed to assess the incidence and predominant location of local recurrences in rectal cancer. The distribution of lymphatic spread was analyzed in another 10 reports to record the relative frequency and location of metastatic lymph nodes in rectal cancer, according to the stage and level of the primary tumor. Results: The mesorectal, posterior, and inferior pelvic subsites are most at risk for local recurrences, whereas lymphatic tumor spread occurs mainly in three directions: upward into the inferior mesenteric nodes; lateral into the internal iliac lymph nodes; and, in a few cases, downward into the external iliac and inguinal lymph nodes. -

Adjuvant Chemo-Radiotherapy in Colorectal Malignancy
STUDY OF ROLE OF NEO – ADJUVANT CHEMO-RADIOTHERAPY IN COLORECTAL MALIGNANCY DISSERTATION SUBMITTED FOR MASTER OF SURGERY (BRANCH I) GENERAL SURGERY DEGREE EXAMINATION MARCH – 2010 THE TAMILNADU Dr. M. G. R. MEDICAL UNIVERSITY CHENNAI Department of General Surgery, Madurai medical college, Government Rajaji Hospital, Madurai CERTIFICATE This is to certify that the dissertation entitled “STUDY OF ROLE OF NEO – ADJUVANT CHEMO-RADIOTHERAPY IN COLORECTAL MALIGNANCY ” submitted by DR.K.VENUGOPALA to the faculty of surgery, The Tamil nadu Dr. M.G.R. Medical university, Chennai in partial fulfillment of the requirement for the award of M.S. Degree in GENERAL SURGERY is a bonafide work carried out by him during the period of April 2008 – November 2009 under my direct supervision and guidance. Place : Madurai Date : Professor and Head Department of General Surgery, Madurai Medical College, Madurai. DECLARATION I, DR.K.VENUGOPALA solemnly declare that the dissertation titled “STUDY OF ROLE OF NEO – ADJUVANT CHEMO-RADIOTHERAPY IN COLORECTAL MALIGNANCY” has been prepared by me. This is submitted to The Tamil Nadu Dr. M.G.R. Medical University, Chennai, in partial fulfillment of the requirement for the award of Master of Surgery, (Branch I) General Surgery Degree Examination to be held in March 2010. Place : Madurai Date : DR.K.VENUGOPALA ACKNOWLEDGEMENT • I Sincerely express my thanks to my unit chief (rtd) Prof.,Dr. V. SeethaRaman M.S., Prof of Principles & Practice of Surgery, (rtd) for his guidance in selecting and encouragement for the study. • I am thankful to Prof, & HOD Dr.S.Gopinath M.S., and also unit incharge for his valuable guidance through my study. -

Pastest Awad Notes Paper 1 2020
PASTEST NOTES 2020- AWAD PAPER 1 (APPLIED BASIC SCIENCE) Contents Anatomy ........................................................................................................................................................ 2 Abdominal Vessels .................................................................................................................................... 2 Abdominal Viscera .................................................................................................................................... 5 Abdominal wall and cavity ...................................................................................................................... 11 CNS, Brain................................................................................................................................................ 12 Lower limb .............................................................................................................................................. 26 Neck ........................................................................................................................................................ 30 Pelvis ....................................................................................................................................................... 33 Upper limb and breast ............................................................................................................................ 36 Spine....................................................................................................................................................... -

Inguinal Lymph Nodes Management in Squamous Cell Carcinoma of the Anal Canal
Article ID: WMC001565 2046-1690 Inguinal Lymph Nodes Management In Squamous Cell Carcinoma Of The Anal Canal Corresponding Author: Dr. Saleh M Abbas, Surgeon, Surgery - Australia Submitting Author: Dr. Saleh M Abbas, Surgeon, Surgery - Australia Article ID: WMC001565 Article Type: Review articles Submitted on:20-Feb-2011, 06:33:08 AM GMT Published on: 22-Feb-2011, 08:50:03 PM GMT Article URL: http://www.webmedcentral.com/article_view/1565 Subject Categories:GENERAL SURGERY Keywords:Carcinoma, Radiotherapy, Inguinal Nodes How to cite the article:Abbas S M. Inguinal Lymph Nodes Management In Squamous Cell Carcinoma Of The Anal Canal . WebmedCentral GENERAL SURGERY 2011;2(2):WMC001565 WebmedCentral > Review articles Page 1 of 9 WMC001565 Downloaded from http://www.webmedcentral.com on 24-Dec-2011, 07:35:37 AM Inguinal Lymph Nodes Management In Squamous Cell Carcinoma Of The Anal Canal Author(s): Abbas S M Abstract Background Current issues in the management of clinically Although Squamous cell carcinoma (SCC) of the anal negative inguinal lymph nodes in squamous cell canal is an uncommon disease it is the most common carcinoma of the anal canal. Quality of the available malignant tumor of the anal canal accounting for 90% evidence. of malignant tumors at this site. Survival is determined Background essentially by two factors; the size of the tumor and The first line of management of squamous cell lymph node spread. Patients with no clinical evidence carcinoma (SCC) of the anal canal is chemo-radiation. of nodal disease have a ten-year survival of 73% The radiation field includes, in addition to the anal compared with 53% in those with nodal disease.1 region and the perirectal nodes, the iliac and both Historically the treatment of SCC of the anal canal was inguinal triangles to target the inguinal nodes. -

Ocular Conjunctival Inoculation of SARS-Cov-2 Can Cause Mild COVID-19 in Rhesus Macaques
ARTICLE https://doi.org/10.1038/s41467-020-18149-6 OPEN Ocular conjunctival inoculation of SARS-CoV-2 can cause mild COVID-19 in rhesus macaques Wei Deng1,3, Linlin Bao1,3, Hong Gao1,3, Zhiguang Xiang1,3, Yajin Qu1,3, Zhiqi Song1,3, Shuran Gong1, Jiayi Liu2, Jiangning Liu1, Pin Yu1, Feifei Qi1, Yanfeng Xu1, Fengli Li1, Chong Xiao1,QiLv1, Jing Xue1, Qiang Wei1, Mingya Liu1, Guanpeng Wang1, Shunyi Wang1, Haisheng Yu1, Ting Chen1, Xing Liu1, Wenjie Zhao1, Yunlin Han1 & ✉ Chuan Qin 1 1234567890():,; Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is highly transmitted through the respiratory route, but potential extra-respiratory routes of SARS-CoV-2 trans- 6 mission remain uncertain. Here we inoculated five rhesus macaques with 1 × 10 TCID50 of SARS-CoV-2 conjunctivally (CJ), intratracheally (IT), and intragastrically (IG). Nasal and throat swabs collected from CJ and IT had detectable viral RNA at 1–7 days post-inoculation (dpi). Viral RNA was detected in anal swabs from only the IT group at 1–7 dpi. Viral RNA was undetectable in tested swabs and tissues after intragastric inoculation. The CJ infected animal had a higher viral load in the nasolacrimal system than the IT infected animal but also showed mild interstitial pneumonia, suggesting distinct virus distributions. This study shows that infection via the conjunctival route is possible in non-human primates; further studies are necessary to compare the relative risk and pathogenesis of infection through these different routes in more detail. 1 NHC Key Laboratory of Human Disease Comparative Medicine, Beijing Key Laboratory for Animal Models of Emerging and Remerging Infectious Diseases, Institute of Laboratory Animal Science, Chinese Academy of Medical Sciences and Comparative Medicine Center, Peking Union Medical College, Beijing, China. -

Integrated Representation of Clinical Data and Medical Knowledge: An
INTEGRATED REPRESENTATION OF CLINICAL DATA AND MEDICAL KNOWLEDGE AN ONTOLOGY-BASED APPROACH FOR THE RADIOLOGY DOMAIN Heiner Oberkampf DISSERTATION for the degree of Doctor of Natural Sciences (Dr. rer. nat.) University of Augsburg Department of Computer Science Software Methodologies for Distributed Systems Software Methodologies for distributed systems October 2015 Integrated Representation of Clinical Data and Medical Knowledge An Ontology-based Approach Supervisor: Prof. Dr. Bernhard L. Bauer, Department of Computer Science, University of Augsburg, Germany Prof. Dr. Elisabeth André, Department of Computer Science, University of Augsburg, Germany Advisor: Prof. Dr. Sonja Zillner, Siemens AG Corporate Technology, Munich, Germany Date of defence of dissertation (oral examination): March 17th, 2016 Copyright © Heiner Oberkampf, Augsburg, October 2015 Contents I. INTRODUCTION, BASICS, RELATED WORK1 1. Introduction3 1.1. Problems and Challenges...........................6 1.2. Objectives and Approach........................... 11 1.3. Publications................................... 16 1.4. Outline...................................... 19 1.5. Acknowledgements.............................. 21 2. Basics 23 2.1. Knowledge Representation.......................... 24 2.1.1. Terminologies, Classification Systems and Ontologies.... 24 2.1.2. Description Logic and Reasoning................. 27 2.1.3. The Semantic Web Technology Stack............... 28 2.2. Biomedical Ontologies............................ 32 2.2.1. Unified Medical Language System................ 33 2.2.2. Open Biological and Biomedical Ontologies.......... 34 2.2.3. Other Ontologies, Classification and Coding Systems.... 38 2.3. Semantic Annotations............................. 40 3. Related Work 41 3.1. The Relation between Data Models and Reference Ontologies.... 42 3.1.1. HL7 Reference Information Model Version 3.......... 44 3.1.2. OpenEHR................................ 46 3.1.3. DICOM-Structured Reporting................... 48 3.1.4. OBO-based Models.........................