A New Anatomically-Preserved Plant from the Lower Devonian of Quebec (Canada): Implications for Euphyllophyte Phylogeny and Early Evolution of Structural Complexity
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Comparative Study of the Primary Vascular System Of
Amer. J. Bot. 55(4): 464-472. 1!16'>. A COMPARATIVE STUDY OF THE PRIMARY VASCULAR SYSTE~1 OF CONIFERS. III. STELAR EVOLUTION IN GYMNOSPERMS 1 KADAMBARI K. NAMBOODIRI2 AND CHARLES B. BECK Department of Botany, University of Michigan, Ann Arbor ABST RAe T This paper includes a survey of the nature of the primary vascular system in a large number of extinct gymnosperms and progymnosperms. The vascular system of a majority of these plants resembles closely that of living conifers, being characterized, except in the most primitive forms which are protostelic, by a eustele consisting of axial sympodial bundles from which leaf traces diverge. The vascular supply to a leaf originates as a single trace with very few exceptions. It is proposed that the eustele in the gyrr.nosperms has evolved directly from the protostele by gradual medullation and concurrent separation of the peripheral conducting tissue into longitudinal sympodial bundles from which traces diverge radially. A subsequent modification results in divergence of traces in a tangential plane, The closed vascular system of conifers with opposite and whorled phyllotaxis, in which the vascular supply to a leaf originates as two traces which subsequently fuse, is considered to be derived from the open sympodial system characteristic of most gymnosperms. This hypothesis of stelar evolution is at variance with that of Jeffrey which suggests that the eustele of seed plants is derived by the lengthening and overlapping of leaf gaps in a siphonostele followed by further reduction in the resultant vascular bundles. This study suggests strongly that the "leaf gap" of conifers and other extant gymnosperms is not homologous with that of siphonostelic ferns and strengthens the validity of the view that Pterop sida is an unnatural group. -

The Lower Devonian Water Canyon Formation of Northeastern Utah
Utah State University DigitalCommons@USU All Graduate Theses and Dissertations Graduate Studies 5-1963 The Lower Devonian Water Canyon formation of Northeastern Utah Michael E. Taylor Follow this and additional works at: https://digitalcommons.usu.edu/etd Part of the Geology Commons Recommended Citation Taylor, Michael E., "The Lower Devonian Water Canyon formation of Northeastern Utah" (1963). All Graduate Theses and Dissertations. 6626. https://digitalcommons.usu.edu/etd/6626 This Thesis is brought to you for free and open access by the Graduate Studies at DigitalCommons@USU. It has been accepted for inclusion in All Graduate Theses and Dissertations by an authorized administrator of DigitalCommons@USU. For more information, please contact [email protected]. THE LOWER DEVONIAN WATER CANYON FORMATION OF NORTHEASTERN UTAH by Michael E. Taylor A thesis submitted in partial fulfillment of the requirements for the degree of MASTER OF SCIENCE in Geology UTAH STATE UNIVERSITY Logan, Utah 1963 ACKNOWLEDGMENTS The writer greatly appreciates the advice and criticism offered by Dean J. Stewart Williams, Dr. Clyde T . Hardy , and Dr. Donald R. Olsen, of the Department of Geology of Utah State University. He is also grateful to his parents for their encouragement during the course of this investigation and for capable assistance with the preparation of the manuscript. Michael E. Taylor TABLE OF CONTENTS Page INTRODUCTION o 1 General statement 1 Geolog ic setting o 3 External stratigraphic relations 4 WATER CANYON FORMATION 8 General statement 8 Card Member 9 Grassy Flat Member 13 Areal distribution 22 Paleonto logy 27 Age 33 Correlat i on 35 Environment of deposition 37 Source of detr itus 40 SUMMARY , 42 LITE RATURE CITED 43 APPENDIX 46 LIST OF FIGURES Figure Page 1. -
The Carboniferous Bowland Shale Gas Study: Geology and Resource Estimation
THE CARBONIFEROUS BOWLAND SHALE GAS STUDY: GEOLOGY AND RESOURCE ESTIMATION The Carboniferous Bowland Shale gas study: geology and resource estimation i © DECC 2013 THE CARBONIFEROUS BOWLAND SHALE GAS STUDY: GEOLOGY AND RESOURCE ESTIMATION Disclaimer This report is for information only. It does not constitute legal, technical or professional advice. The Department of Energy and Climate Change does not accept any liability for any direct, indirect or consequential loss or damage of any nature, however caused, which may be sustained as a result of reliance upon the information contained in this report. All material is copyright. It may be produced in whole or in part subject to the inclusion of an acknowledgement of the source, but should not be included in any commercial usage or sale. Reproduction for purposes other than those indicated above requires the written permission of the Department of Energy and Climate Change. Suggested citation: Andrews, I.J. 2013. The Carboniferous Bowland Shale gas study: geology and resource estimation. British Geological Survey for Department of Energy and Climate Change, London, UK. Requests and enquiries should be addressed to: Toni Harvey Senior Geoscientist - UK Onshore Email: [email protected] ii © DECC 2013 THE CARBONIFEROUS BOWLAND SHALE GAS STUDY: GEOLOGY AND RESOURCE ESTIMATION Foreword This report has been produced under contract by the British Geological Survey (BGS). It is based on a recent analysis, together with published data and interpretations. Additional information is available at the Department of Energy and Climate Change (DECC) website. https://www.gov.uk/oil-and-gas-onshore-exploration-and-production. This includes licensing regulations, maps, monthly production figures, basic well data and where to view and purchase data. -

The Origin and Early Evolution of Vascular Plant Shoots and Leaves Rstb.Royalsocietypublishing.Org C
Downloaded from http://rstb.royalsocietypublishing.org/ on January 22, 2018 The origin and early evolution of vascular plant shoots and leaves rstb.royalsocietypublishing.org C. Jill Harrison 1 and Jennifer L. Morris 2 1School of Biological Sciences, and 2School of Earth Sciences, University of Bristol, 24 Tyndall Avenue, Bristol BS8 1TQ, UK Review CJH, 0000-0002-5228-600X; JLM, 0000-0002-7453-3841 Cite this article: Harrison CJ, Morris JL. 2017 The morphology of plant fossils from the Rhynie chert has generated long- standing questions about vascular plant shoot and leaf evolution, for The origin and early evolution of vascular plant instance, which morphologies were ancestral within land plants, when did shoots and leaves. Phil. Trans. R. Soc. B 373 : vascular plants first arise and did leaves have multiple evolutionary origins? 20160496. Recent advances combining insights from molecular phylogeny, palaeobotany http://dx.doi.org/10.1098/rstb.2016.0496 and evo–devo research address these questions and suggest the sequence of morphological innovation during vascular plant shoot and leaf evolution. The evidence pinpoints testable developmental and genetic hypotheses relat- Accepted: 11 August 2017 ing to the origin of branching and indeterminate shoot architectures prior to the evolution of leaves, and demonstrates underestimation of polyphyly in One contribution of 18 to a discussion meeting the evolution of leaves from branching forms in ‘telome theory’ hypotheses issue ‘The Rhynie cherts: our earliest terrestrial of leaf evolution. This review discusses fossil, developmental and genetic ecosystem revisited’. evidence relating to the evolution of vascular plant shoots and leaves in a phylogenetic framework. This article is part of a discussion meeting issue ‘The Rhynie cherts: our Subject Areas: earliest terrestrial ecosystem revisited’. -

Getting to the Roots: a Developmental Genetic View of Root Anatomy and Function from Arabidopsis to Lycophytes
fpls-09-01410 September 21, 2018 Time: 17:3 # 1 REVIEW published: 25 September 2018 doi: 10.3389/fpls.2018.01410 Getting to the Roots: A Developmental Genetic View of Root Anatomy and Function From Arabidopsis to Lycophytes Frauke Augstein and Annelie Carlsbecker* Department of Organismal Biology, Physiological Botany and Linnean Centre for Plant Biology in Uppsala, Uppsala University, Uppsala, Sweden Roots attach plants to the ground and ensure efficient and selective uptake of water and nutrients. These functions are facilitated by the morphological and anatomical structures of the root, formed by the activity of the root apical meristem (RAM) and consecutive patterning and differentiation of specific tissues with distinct functions. Despite the importance of this plant organ, its evolutionary history is not clear, but fossils suggest that roots evolved at least twice, in the lycophyte (clubmosses and their allies) and in the euphyllophyte (ferns and seed plants) lineages. Both lycophyte and euphyllophyte roots grow indeterminately by the action of an apical meristem, which is protected by a root cap. They produce root hairs, and in most species the vascular stele is Edited by: guarded by a specialized endodermal cell layer. Hence, most of these traits must have Annette Becker, evolved independently in these lineages. This raises the question if the development Justus Liebig Universität Gießen, Germany of these apparently analogous tissues is regulated by distinct or homologous genes, Reviewed by: independently recruited from a common ancestor of lycophytes and euphyllophytes. Hongchang Cui, Currently, there are few studies of the genetic and molecular regulation of lycophyte Florida State University, United States and fern roots. -

Heterospory: the Most Iterative Key Innovation in the Evolutionary History of the Plant Kingdom
Biol. Rej\ (1994). 69, l>p. 345-417 345 Printeii in GrenI Britain HETEROSPORY: THE MOST ITERATIVE KEY INNOVATION IN THE EVOLUTIONARY HISTORY OF THE PLANT KINGDOM BY RICHARD M. BATEMAN' AND WILLIAM A. DiMlCHELE' ' Departments of Earth and Plant Sciences, Oxford University, Parks Road, Oxford OXi 3P/?, U.K. {Present addresses: Royal Botanic Garden Edinburiih, Inverleith Rojv, Edinburgh, EIIT, SLR ; Department of Geology, Royal Museum of Scotland, Chambers Street, Edinburgh EHi ijfF) '" Department of Paleohiology, National Museum of Natural History, Smithsonian Institution, Washington, DC^zo^bo, U.S.A. CONTENTS I. Introduction: the nature of hf^terospon' ......... 345 U. Generalized life history of a homosporous polysporangiophyle: the basis for evolutionary excursions into hetcrospory ............ 348 III, Detection of hcterospory in fossils. .......... 352 (1) The need to extrapolate from sporophyte to gametophyte ..... 352 (2) Spatial criteria and the physiological control of heterospory ..... 351; IV. Iterative evolution of heterospory ........... ^dj V. Inter-cladc comparison of levels of heterospory 374 (1) Zosterophyllopsida 374 (2) Lycopsida 374 (3) Sphenopsida . 377 (4) PtiTopsida 378 (5) f^rogymnospermopsida ............ 380 (6) Gymnospermopsida (including Angiospermales) . 384 (7) Summary: patterns of character acquisition ....... 386 VI. Physiological control of hetcrosporic phenomena ........ 390 VII. How the sporophyte progressively gained control over the gametophyte: a 'just-so' story 391 (1) Introduction: evolutionary antagonism between sporophyte and gametophyte 391 (2) Homosporous systems ............ 394 (3) Heterosporous systems ............ 39(1 (4) Total sporophytic control: seed habit 401 VIII. Summary .... ... 404 IX. .•Acknowledgements 407 X. References 407 I. I.NIRODUCTION: THE NATURE OF HETEROSPORY 'Heterospory' sensu lato has long been one of the most popular re\ie\v topics in organismal botany. -

Conifer Reproductive Biology Claire G
Conifer Reproductive Biology Claire G. Williams Conifer Reproductive Biology Claire G. Williams USA ISBN: 978-1-4020-9601-3 e-ISBN: 978-1-4020-9602-0 DOI: 10.1007/978-1-4020-9602-0 Springer Dordrecht Heidelberg London New York Library of Congress Control Number: 2009927085 © Springer Science+Business Media B.V. 2009 No part of this work may be reproduced, stored in a retrieval system, or transmitted in any form or by any means, electronic, mechanical, photocopying, microfilming, recording or otherwise, without written permission from the Publisher, with the exception of any material supplied specifically for the purpose of being entered and executed on a computer system, for exclusive use by the purchaser of the work. Cover Image: Snow and pendant cones on spruce tree (reproduced with permission of Photos.com). Printed on acid-free paper Springer is part of Springer Science+Business Media (www.springer.com) Foreword When it comes to reproduction, gymnosperms are deeply weird. Cycads and coni- fers have drawn out reproduction: at least 13 genera take over a year from pollina- tion to fertilization. Since they don’t apparently have any selection mechanism by which to discriminate among pollen tubes prior to fertilization, it is natural to won- der why such a delay in reproduction is necessary. Claire Williams’ book celebrates such oddities of conifer reproduction. She has written a book that turns the context of many of these reproductive quirks into deeper questions concerning evolution. The origins of some of these questions can be traced back Wilhelm Hofmeister’s 1851 book, which detailed the revolutionary idea of alternation of generations. -
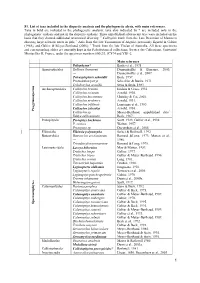
S1. List of Taxa Included in the Disparity Analysis and the Phylogenetic Alysis, with Main References
S1. List of taxa included in the disparity analysis and the phylogenetic alysis, with main references. Taxa in bold are included in the phylogenetic analysis; taxa also indicated by * are included only in the phylogenetic analysis and not in the disparity analysis. Three unpublished arborescent taxa were included on the basis that they showed additional anatomical diversity. 1 Callixylon trunk from the Late Devonian of Marrocco showing large sclerotic nests in pith; 2 Axis from the late Tournaisian of Algeria, previously figured in Galtier (1988), and Galtier & Meyer-Berthaud (2006); 3 Trunk from the late Viséan of Australia. All these specimens and corresponding slides are currently kept in the Paleobotanical collections, Service des Collections, Université Montpellier II, France, under the specimen numbers 600/2/3, JC874 and YB1-2. Main reference Psilophyton* Banks et al., 1975 Aneurophytales Rellimia thomsonii Dannenhoffer & Bonamo, 2003; --- Dannenhoffer et al., 2007. Tetraxylopteris schmidtii Beck, 1957. Proteokalon petryi Scheckler & Banks, 1971. Triloboxylon arnoldii Stein & Beck, 1983. s m Archaeopteridales Callixylon brownii Hoskin & Cross, 1951. r e Callixylon erianum Arnold, 1930. p s o Callixylon huronensis Chitaley & Cai, 2001. n Callixylon newberry Arnold, 1931. m y g Callixylon trifilievii Lemoigne et al., 1983. o r Callixylon zalesskyi Arnold, 1930. P Callixylon sp. Meyer-Berthaud, unpublished data1. Eddya sullivanensis Beck, 1967. Protopityales Protopitys buchiana Scott, 1923; Galtier et al., 1998. P. scotica Walton, 1957. Protopitys sp. Decombeix et al., 2005. Elkinsiales Elkinsia polymorpha Serbet & Rothwell, 1992. Buteoxylales Buteoxylon gordonianum Barnard &Long, 1973; Matten et al., --- 1980. Triradioxylon primaevum Barnard & Long, 1975. Lyginopteridales Laceya hibernica May & Matten, 1983. Tristichia longii Galtier, 1977. -

SYSTÉMATIQUE DES EMBRYOPHYTES Document Illustrant Plus Particulièrement Les Taxons Européens Version 4 Octobre 2007
Université Pierre et Marie Curie (PARIS 6) Préparation à l’agrégation SVSTU, secteur B SYSTÉMATIQUE DES EMBRYOPHYTES Document illustrant plus particulièrement les taxons européens version 4 octobre 2007 Catherine Reeb, Préparation agrégation SVSTU, UPMC Jean-Yves Dubuisson, Laboratoire Paléobotanique et Paléoécologie, équipe Paléodiversité, systématique et évolution des Embryophytes, UPMC Dessin de couverture : extrait d’un traité botanique tibétain Pour Garance Remerciements: merci à Jean et Monique Duperon pour les clés de détermination des familles d’Angiospermes, à A.M pour la reproduction de couverture, à Michaël Manuel et Eric Queinnec pour la partie I de l’introduction. Egalement à tous les illustrateurs qui ont autorisé l’utilisation de leurs documents mis en ligne sur Internet : Françoise Gantet, Prof. I. Foissner, Association Endemia de Nouvelle Calédonie. 1 Table des matières Introduction 3 Les données essentielles pour l’agrégation . 5 I Systématique générale des Embryophytes 6 I.1 Caractères des Embryophytes . 6 I.2 La place des Embryophytes au sein de la lignée verte . 7 I.3 Le groupe frère des Embryophytes . 7 I.4 Relations au sein des Embryophytes :les points acquis aujourd’hui . 8 I.4.1 Marchantiophytes, Bryophytes, Anthocérotophytes à la base des Embryophytes . 8 I.4.2 Les Trachéophytes ou plantes vasculaires, un groupe monophylétique . 9 I.4.3 Les Spermatophytes ou plantes à ovules, un groupe monophylétique . 9 I.5 Quelques points encore en discussion . 11 I.5.1 Position et relations des trois clades basaux (Bryophytes, Marchantiophytes et Anthocérotophytes) 11 I.5.2 Relations au sein des Spermatophytes :l’hypothèse "Gnepine" . 13 II Les principaux clades d’Embryophytes 15 II.1 MARCHANTIOPHYTA:Les Marchantiophytes ou Hépatiques . -

System Klasyfikacji Organizmów
1 SYSTEM KLASYFIKACJI ORGANIZMÓW Nie ma dziś ogólnie przyjętego systemu klasyfikacji organizmów. Nie udaje się osiągnąć zgodności nie tylko w odniesieniu do zakresów i rang poszczególnych taksonów, ale nawet co do zasad metodologicznych, na których klasyfikacja powinna być oparta. Zespołowe dzieła przeglądowe z reguły prezentują odmienne i wzajemnie sprzeczne podejścia poszczególnych autorów, bez nadziei na consensus. Nie ma więc mowy o skompilowaniu jednolitego schematu klasyfikacji z literatury i system przedstawiony poniżej nie daje nadziei na zaakceptowanie przez kogokolwiek poza kompilatorem. Przygotowany został w oparciu o kilka zasad (skądinąd bardzo kontrowersyjnych): (1) Identyfikowanie ga- tunków i ich klasyfikowanie w jednostki rodzajowe, rodzinowe czy rzędy jest zadaniem specjalistów i bez szcze- gółowych samodzielnych studiów nie można kwestionować wyników takich badań. (2) Podział świata żywego na królestwa, typy, gromady i rzędy jest natomiast domeną ewolucjonistów i dydaktyków. Powody, które posłużyły do wydzielenia jednostek powinny być jasno przedstawialne i zrozumiałe również dla niespecjalistów, albowiem (3) podstawowym zadaniem systematyki jest ułatwianie laikom i początkującym badaczom poruszanie się w obezwładniającej złożoności świata żywego. Wątpliwe jednak, by wystarczyło to do stworzenia zadowalającej klasyfikacji. W takiej sytuacji można jedynie przypomnieć, że lepszy ułomny system, niż żaden. Królestwo PROKARYOTA Chatton, 1938 DNA wyłącznie w postaci kolistej (genoforów), transkrypcja nie rozdzielona przestrzennie od translacji – rybosomy w tym samym przedzia- le komórki, co DNA. Oddział CYANOPHYTA Smith, 1938 (Myxophyta Cohn, 1875, Cyanobacteria Stanier, 1973) Stosunkowo duże komórki, dwuwarstwowa błona (Gram-ujemne), wewnętrzna warstwa mureinowa. Klasa CYANOPHYCEAE Sachs, 1874 Rząd Stigonematales Geitler, 1925; zigen – dziś Chlorofil a na pojedynczych tylakoidach. Nitkowate, rozgałęziające się, cytoplazmatyczne połączenia mię- Rząd Chroococcales Wettstein, 1924; 2,1 Ga – dziś dzy komórkami, miewają heterocysty. -

Dicranophyllum Glabrum (DAWSON) STOPES, an UNUSUAL ELEMENT of LOWER WESTPHALIAN FLORAS in ATLANTIC CANADA
DICRANOPHYLLUM GLABRUM OF LOWER WESTPHALIAN FLORAS IN CANADA 7 Dicranophyllum glabrum (DAWSON) STOPES, AN UNUSUAL ELEMENT OF LOWER WESTPHALIAN FLORAS IN ATLANTIC CANADA Robert H. WAGNER Centro Paleobotánico, Jardín Botánico de Córdoba, Avda. de Linneo, s/n, 14004 Córdoba (Spain); e-mail: [email protected] Wagner, R. H. 2005. Dicranophyllum glabrum (Dawson) Stopes, an unusual element of lower Westphalian fl oras in Atlantic Canada. [Dicranophyllum glabrum (Dawson) Stopes, un elemento raro de las fl oras del Westfaliense inferior del Canadá Atlántico.] Revista Española de Paleontología, 20 (1), 7-13. ISSN 0213-6937. ABSTRACT Rare but well preserved repeatedly dichotomised leaves, apparently in a single plane, are identifi ed with Dicrano- phyllum, an unusual gymnosperm attributed to a special order, the Dicranophyllales. The specimens recorded here from the “Fern Ledges” at Saint John, New Brunswick are from lower Westphalian (Langsettian) strata, which is a low horizon for this genus, which is best known from the Stephanian and Lower Permian. Comparison is made with various species described from the Carboniferous in Europe. Keywords: Dicranophyllum, Langsettian, New Brunswick, Nova Scotia. RESUMEN Se han hallado ejemplares aislados con hojas que se dicotomizan repetidamente en lo que parece ser un solo plano en estratos del Westfaliense inferior (Langsettiense) de los “Fern Ledges” en Saint John, Nueva Brunswick. Estos ejemplares se han atribuido al género Dicranophyllum, una gimnosperma del orden de las Dicranophy llales. Se trata de un registro antiguo para este género, que se conoce sobre todo en materiales del Estefaniense y del Pérmico Inferior. Se compara con varias de las especies descritas del Carbonífero de Europa. -

Lyginopteris Oldhamia Class Cycadopsida Order Pteridospermales Family Lyginopteridaceae Genus Lyginopteris Species L.Oldhamia
Prepared for B.Sc. Part II Botany Hons. by Prof.(Dr.) Manorma Kumari Lyginopteris oldhamia Class Cycadopsida Order Pteridospermales Family Lyginopteridaceae Genus Lyginopteris Species L.oldhamia Lyginopteris is well known genus in the family Lyginopteridaceae. It was earlier known as Calymmatotheca hoeninghausii. Williamson, Scott, Brongniart, Binney, Potonie and Oliver and Scott described this genus in detail. It was found in abundance in the coal ball horizon of Lancashire and Yorkshire. Various parts of the plants were discovered and named separately. They were later found to be different organs of the same plants Lyginopteris due to presence of similar type of capitate gland on them (except roots). Various parts are: Leaves Sphenopteris hoeninghausii Stems Lyginopteris oldhamia Petioles Rachiopteris aspera Roots Kaloxylon hookeri Seeds Lagenostoma lomaxi Morphological Features The stem of Lyginopteris oldhamia was long aerial and radially symmetrical varying in diameter from 2mm to 4cm. It was frequently branched and bore adventitious roots that were probably prop roots that supported the weak stems. The leaves were spirally arranged and up to 0.5 metre long. The leaves were bipinnate to tripinnate. The pinnae had pinnules or leaflets and were present at right angles to the rachis. The rachis and petiole were studded with capitate glands. Lyginopteris oldhamia: A. Showing external character; B. Frond bearing pollen sac; C. Frond bearing seeds. 2 Anatomy Stem: The transverse sections of the stem show nearly circular outline. Outer layer is epidermis, next to it is many layered cortex. Outer cortex contains radially broadened fibrous strands that forms a vertical network. It provides mechanical strength to the weak stem.