May 2011 Newsletter
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Update from the CEO by Charles Jaffe, MD, Phd, HL7 CEO HL7’S 22Nd Annual Plenary Meeting
AUGUST 2008 In This Issue... Update from the CEO By Charles Jaffe, MD, PhD, HL7 CEO HL7’s 22nd Annual Plenary Meeting..... 2 Work Group Co-Chair Elections: As the languid days of August almost impercep- Development Organizations What to Expect in Vancouver................ 3 tibly transform into the cool autumn nights of (SDOs). Most importantly, September, some significant milestones emerged Update from Headquarters.............. 4-5 it is a living document. The from the HL7 landscape. Roadmap development team ExL Pharma’s 4th Annual EHR and will continue to be com- Charles Jaffe, MD, PhD eClinical Technologies Conference....... 6 The membership of HL7 has its very first posed of a broad constitu- News from the PMO.............................. 6 Roadmap. A strategic plan for technical and busi- ency with distinct requirements, and with plans to ness development is embodied in this document. It publish Roadmap updates annually. Version 3 Normative Edition 2008 Now Available...................................... 7 is more than just a promise to our stakeholders; the Roadmap provides the end-user community, gov- As the Roadmap matures, it will reflect the changing Multiple Modes of Delivering ernment agencies, software developers and other Education............................................. 8 business needs of our organization. More opportuni- standards development organizations clear guide- ties require more creative resource development and Advisory Council News....................... 9 lines for our products and services. In addition, the new means of expanding our funding model. Co-Chair Election Results.................... 9 Roadmap offers something beyond a commitment to develop a new standard or a new release of a Part of our new funding model emerged with the HL7 Certification Exam balloted one. -

HL7 Mobile Health Work Group
SEPTEMBER 2013 HL7 Mobile Health Work Group 101 By Gora Datta, Co-Chair, HL7 Mobile Health Work Group; Co-Lead, HL7 In- teroperability EHR Work Group; HL7 Ambassador; Group Chairman & CEO, CAL2CAL Corporation The HL7 Mobile Health (MH) Work Group is now • Identify and one year old! Over the past year, the work group promote mobile (WG) has rapidly matured from loosely held health concepts interested individuals to a cohesive group of ex- for interoper- perts that meets regularly. We welcome one and ability as Gora Datta all to join our Mobile Friday weekly calls every adopted and Friday at 11am ET. adapted for use in the mobile environment. • Coordinate and cooperate with other The group’s mission “The HL7 Mobile Health groups interested in using mobile health to Work Group creates and promotes health infor- promote health, wellness, public health, mation technology standards and frameworks for clinical, social media, and other settings. mobile health” captures the essence of its charter, • Provide a forum where HL7 members and as outlined: stakeholders collaborate in standardizing • Identify (and develop, as applicable) data to enable the secure exchange, storage, standards and functional requirements that analysis, and transmission of data and are specific to the mobile health environment. information for mobile applications and/ or mobile devices. One of the challenges the group has is that it is one of the few HL7 groups (like the EHR WG) that is not domain specific; and is more horizontal in nature. Therefore, its charter cuts across other vertical/domain oriented (work) groups. Given the tremendous interest and participation not only in the US but also globally, the HL7 Mobile Health WG is rapidly stepping up to identify and fill the gaps in the mobile health standards space. -

Healthcare Industry Highlight: Revenue Cycle Management
Industry Highlight: Revenue Cycle Management Q1 2019 RCM Overview Intelligent, Automated Workflow Patient Access Claims Management RCM intelligently automates the complex tasks of the front and back office, optimizing: Case Management ✓ Patient encounters and consolidation of REVENUE records Charges & CYCLE ✓ Participation in value-based programs to Reimbursement generate maximum revenue ✓ Claims management, accounts receivable and claim resubmission Clinical ✓ Medical practice workflow, reducing Documentation redundant staff effort Medical Coding Increasing complexity in medical coding and rising healthcare costs make RCM an essential tool to maintain cash flow and stay solvent 1 RCM Market Size and Outlook The global RCM market is projected to grow to $65.2BN by 2025 from $23.6BN in 2016, a nine-year CAGR of 12.0% Value of Global RCM Market ($USD in BN) $80 Facing tight margins, time-consuming 60 regulation and enormous waste in the healthcare system, medical practices 40 are looking for adaptable solutions to streamline workflow, generating 20 significant drive in the RCM market 0 2016 2017 2018 2019 2020 2021 2022 2023 2024 2025 $23.6BN $16.5BN 40% Value of global RCM market in Estimated hospital spend on Percentage of RCM end-user market 2016 external RCM by 2020 constituted by physicians’ offices 12.0% $6.7BN 3-5% CAGR through 2025 Annual provider spend on Hospitals’ lost revenues due RCM to RCM errors 2 Source: CMS NHE Projections , Grandview Research, MicroMarketMonitor, HFMA Inefficiencies RCM Can Help Solve Key Industry Trends: -

Seven Members Honored HL7 Honored Seven Members with the 14Th Annual W
JANUARY 2011 Upcoming WORKING GROUP MEETINGS 2010 Ed Hammond Volunteer of the Year Awards Seven members honored HL7 honored seven members with the 14th annual W. Edward Hammond, PhD Volunteer of the Year Award. Established in 1997, the award is named after Dr. Ed Hammond, one of HL7’s most active volunteers, founding member and past Board chair. The award recognizes individuals who January 9 – 14, 2011 May 15 – 20, 2011 have made significant contributions to HL7’s success. The 2010 recipients include: • Hugh Glover and Julie James, HL7 UK January Working Group • Stan Huff, MD, chief medical informatics officer, Intermountain Healthcare Working Group Meeting • Charlie Mead, MD, MSc, CTO, 3rd Millennium, Inc. Meeting • Mark Shafarman, principal, Shafarman Consulting Hilton in the Walt Disney World Resort • D. Mead Walker, Health Data and Interoperability, Inc. Cliftons Meeting and Training Center Lake Buena Vista, FL • Pat Van Dyke, director of information, security, privacy and EDI representing Delta Dental and the Amora Hotel Plans Association Sydney, Australia About the Volunteers: Hugh Glover and Julie James have been involved with HL7 since 2001. They received the award jointly as they are partners both personally and profession- ally. They have both actively contributed to HL7 for many years and have held leadership positions in the Pharmacy Work Group such as a co-chair or facili- tator. Their backgrounds—James as a pharmacist and Glover’s expertise in data modeling—complement each other and have brought valuable insight to HL7. Both have been involved with Version 3 development since its inception. James September 11 – 16, 2011 January 15 – 20, 2012 is also active in the Patient Safety Work Group. -
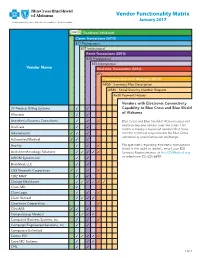
Vendor Functionality Matrix January 2017 an Independent Licensee of the Blue Cross and Blue Shield Association
Vendor Functionality Matrix January 2017 An Independent Licensee of the Blue Cross and Blue Shield Association Readiness Validated Claims Transactions (5010) 837 Professional 837 Institutional Remit Transactions (5010) 835 Professional 835 Institutional Vendor Name Real-time Transactions (5010) 27x Proprietary Real-time Messages (5010) AB50 - Summary Plan Description AB80 - Social Security Number Request Ax20 Payment History Vendors with Electronic Connectivity 2K Medical Billing Systems 3 3 Capability to Blue Cross and Blue Shield of Alabama Allscripts 3 3 Anesthesia Business Consultants 3 3 3 Blue Cross and Blue Shield of Alabama does not endorse any one vendor over the other. This AnviCare 3 3 matrix is merely a record of vendors that have Athenahealth 3 3 3 met the technical requirements for Blue Cross connectivity and information exchange. Automated Medical 3 3 3 Availity 3 3 3 For questions regarding electronic transactions listed in the table to the left, email your EDI Avid Anesthesiology Solutions 3 3 3 3 3 Services Representative at [email protected] AXIOM Systems Inc. 3 3 or telephone 205-220-6899. BrickMed, LLC 3 3 C&S Research Corporation 3 3 3 3 CBIZ MMP 3 3 Change Healthcare 3 3 3 3 3 3 Claim.MD 3 ClaimLogic 3 3 3 Claim Remedi 3 3 3 3 Clearwave Corporation 3 ClinixMIS 3 3 3 CompuGroup Medical 3 3 3 3 Compulink Business Systems, Inc. 3 3 3 Computer Engineered Solutions, Inc. 3 3 Computers Unlimited Cortex EDI 3 3 3 3 3 Cove MD Systems 3 3 3 CPU 3 3 1 of 4 Readiness Validated Claims Transactions (5010) 837 Professional 837 Institutional Remit Transactions (5010) 835 Professional 835 Institutional Vendor Name Real-time Transactions (5010) 27x Proprietary Real-time Messages (5010) AB50 - Summary Plan Description AB80 - Social Security Number Request Ax20 Payment History Creative Concepts in Communications 3 3 Custom Software Systems, Inc. -

Open Platform Architecture Based Upon SMART Apps Platform and the HL7 FHIR® (DSTU) Demonstrated at HIMSS14
MAY 2014 Open Platform Architecture Based Upon SMART Apps Platform and the HL7 FHIR® (DSTU) Demonstrated at HIMSS14 By David Kreda, Translation Advisor, and Joshua Mandel MD, Lead Architect, SMART Platforms Project Harvard Medical School/Children’s Hospital Boston www.smartplatforms.org During the HIMSS14 Annual Conference (Feb- ferent internal architectures ruary 23-27, 2014 in Orlando, Florida), several and technologies, they healthcare IT exhibitors presented an eye-open- were nonetheless able to ing example of the potential of HL7’s newest supply the data via FHIR standard, FHIR® (Fast Healthcare Interoperabil- API calls made by the suite David Kreda ity Resources). The list of exhibitors included of SMART Apps that were Cerner, Intermountain Healthcare, Hewlett Pack- installed on their systems. ard, and Harris Corporation. They presented the Harris Corporation then SMART on FHIR Open Platform Architecture, demonstrated its services which combines FHIR and the open source, web- oriented architecture (SOA) standards based technology stack created by the service, which accepted SMART Platforms Project, a research effort based same FHIR API queries at Harvard Medical School and Children’s Hos- from the SMART apps, fed- pital Boston that enables real-time integration of erated queries to the same substitutable medical apps, or SMART apps, on EHR systems holding the EHR systems. patient data, and synthe- Josh Mandel, MD sized a cross system, longi- Three sophisticated EHR systems, including tudinal patient record, which was returned to the Cerner Millennium, HELP2 from Intermountain requesting SMART app. Healthcare, and VistA from the Veterans Health Administration, were each independently FHIR- Hewlett Packard, which engineered the SMART enabled in less than six weeks. -

Spring 2019 Medical Practice Management
SPRING 20CUSTOMER SUCCESS 19REPORT MEDICAL PRACTICE MANAGEMENT SOFTWARE CATEGORY MEDICAL PRACTICE MANAGEMENT SOFTWARE OVERVIEW Medical practice management software (PMS) is designed to assist medical offices of all sizes operate more efficiently. Mostly, small and medium-size practices utilize a PMS to manage daily administrative and financial functions, and integrate it with electronic medical records The capabilities of PMS include: entering and monitoring patients, storing patient demographics, scheduling patient appointments, handling charge capture, executing billing procedures, presenting insurance claims, processing payments from third parties, insurance providers, and patients, and producing reports for employees. Medical PMS can be used to store and maintain patient records as well as manage large data sets, including details of physicians and other health care providers, medical facilities, insurance companies, procedures, and ICD codes. 2 CUSTOMER SUCCESS Company Score is affected by the following: SCORING METHODOLOGY 1. Number of employees (based on social media and public resources) The FeaturedCustomers.com Customer 2. Vendor momentum based on web traffic and Success score is based on data from our search trends customer success content platform, social 3. Employee satisfaction and engagement presence, as well as additional data aggregated (based on social network ratings) from online sources and social media properties. 4. % traffic increase to your Customer Our ranking engine applies an algorithm to all of References the data collected to calculate the overall 5. Lower Funnel SEO Key Term Rankings Customer Success score. The overall Customer Success score is a weighted average based on 3 CUSTOMER SUCCESS AWARDS parts: Market Leader (90 - 100) Content Score is affected by the following: Vendor on FeaturedCustomers.com 1. -

A Survey of U.S. Healthcare IT Industry Landscape
July 28, 2016 Industry Research Department, Mizuho Bank Mizuho Industry Focus Vol. 183 A Survey of U.S. Healthcare IT Industry Landscape Global Corporate Advisory Americas Tim Wang, CFA, 2016 [email protected] 〈Summary〉 ○ U.S. Healthcare IT industry is booming. Over the recent years, many companies have had robust growth and the sector has attracted huge inflow of private and public investments. There are many types of HCIT companies, operating with different business models and participating in various segments of HCIT. In this report, we try to first identify the drivers behind HCIT growth and then delineate the dynamics in each HCIT segment. ○ Key drivers of HCIT are also the underlying drivers of the U.S. healthcare system, namely Obamacare, the transition to value-based reimbursement model, rising consumerism, industry consolidation, etc. Within HCIT, major drivers include the HITECH Act, advancement of technology, and the convergence of healthcare, IT, and consumer industries. ○ The booming HCIT market is in transition. Incentives for EHR created by the HITECH Act have largely run their course. In the future, the marketplace will be driven less by government subsidies and more by private spending. To earn revenues from private payers, HCIT companies increasingly need to demonstrate the clinical and financial value of their offerings. To achieve this, HCIT companies need to collaborate closely with their customers. ○ For the various HCIT segments, we see three especially promising areas – integration of remote patient monitoring either through wearables or other devices with data analytics and intervention capabilities; big data analytics by Artificial Intelligence; and telemedicine. We expect stable growth for RCM, PM, payer solution segments. -

Clinical Integration Through Electronic Data Interchange
VENDOR LIST Clinical Integration Through Electronic Data Interchange As of February 2021 As of February 2021 Vendor Name Vendor Name Vendor Name 4Medica CareHere-Champ IT Solutions DaVinci Technology Accumedic Computer Systems CareSpan USA Inc DaVita ACE Health Solutions, LLC Cascades Clinical Systems, Inc. DavLong Business Solutions, LLC Acumen Physician Solutions Centura Health Corporate DigiDMS Acurus Solutions Cerner Corp Digital MD Systems ADL Data Systems Cerner PowerChart DocComply Advanced Data Systems Corp Change Healthcare Doctor On Demand Advanced Technologies Group ChartLogic Doc-tor.com AdvancedMD Software ChartPerfect, Inc DoctorWellington Agastha ChenMed DocuTrac Ageology, Inc. ChiroTouch Dr J Exceptional Medicine AllegianceMD Software Inc ClaimPower, Inc. DrChrono AllMeds, Inc ClinicTracker JAG Drscribe Allscripts Clinlab DrugPak LLC American Medical Software Clovi Echo Group American Medical Solutions CoCENTRIX eClinicalWorks Anagen Systems CodoniX eData Platform Angel Systems, Inc. Community Computer Service, Inc. EHealthLine.com, Inc. Ankhos Oncology Software, LLC Complete Healthcare Solutions, Inc eHealthObjects, Inc AntWorks Complete Medical Solutions, Inc. EHR Your Way AP Easy Software Solutions CompuGroup Medical Elation Health Apex Energetics CompuLink Business Systems ELEKTA IMPAC Medical Systems Apex Healthware Computalogic Ellkay Aprima Comtron eMD Office.com Aragon Development Conceptual Mindworks eMDs Arise Lab, LLC Confluence Health Systems LLC eMedical Fusion Artisan Medical Solutions Connexin Software, Inc eMedicalNotes ASP.MD Core Solutions Emedpractice Aspyra Inc. CorEMR Empower Health Services, LLC Athena Health Cority Enable Healthcare Inc. AtlasMD CorrecTek EnableDoc Axion Health Credible Behavioral Health Software Encite Azalea Health Innovations, Inc. Criterions Ensoftek, Inc. AZZLY Crowell Systems Epic Systems Corporation BestNotes CureMD Essential Health Solutions BIZMATICS, Inc Custom Computing Corp Evident Brilogy CyberMed, LLC. -

Clinical Integration Through Electronic Data Interchange
VENDOR LIST Clinical Integration Through Electronic Data Interchange As of July 2021 As of July 2021 Vendor Name Vendor Name Vendor Name 4Medica CareCloud DataLink LLC Accumedic Computer Systems CareHere-Champ IT Solutions DataTel Solutions ACE Health Solutions, LLC CareSpan USA Inc DaVinci Technology Acumen Physician Solutions Cascades Clinical Systems, Inc. DaVita Acurus Solutions Centura Health Corporate DavLong Business Solutions, LLC ADL Data Systems Cerner Corp DigiDMS Advanced Data Systems Corp Change Healthcare Digital MD Systems Advanced Technologies Group ChartLogic DocComply AdvancedMD Software ChartPerfect, Inc Doctor On Demand Agastha ChenMed DoctorWellington Ageology, Inc. ChiroTouch DocuTrac AllegianceMD Software Inc ClaimPower, Inc. Dr J Exceptional Medicine AllMeds, Inc ClinicTracker JAG DrChrono Allscripts Clinlab Drscribe American Medical Software Clovi DrugPak LLC American Medical Solutions CoCENTRIX Echo Group Anagen Systems CodoniX eClinicalWorks Angel Systems, Inc. Community Computer Service, Inc. eData Platform Ankhos Oncology Software, LLC Complete Healthcare Solutions, Inc EHealthLine.com, Inc. AntWorks Complete Medical Solutions, Inc. eHealthObjects, Inc AP Easy Software Solutions CompuGroup Medical EHR Your Way Apex Energetics CompuLink Business Systems Elation Health Apex Healthware Computalogic ELEKTA IMPAC Medical Systems Aprima Computer Service & Support Ellkay Aragon Development Comtron eMD Office.com Arise Lab, LLC Conceptual Mindworks eMDs Artisan Medical Solutions Confluence Health Systems LLC eMedical -

Spring 2018 Medical Practice Management
SPRING 20CUSTOMER SUCCESS 18REPORT MEDICAL PRACTICE MANAGEMENT SOFTWARE CATEGORY MEDICAL PRACTICE MANAGEMENT SOFTWARE OVERVIEW Medical Practice Management software is an integral part of medical practice and is great for assisting in the day to day administrative and financial operations of medical practice. This particular category of software allows users to complete a multitude of tasks ranging from billing and scheduling to generating reports. Medical practice software usually integrates with EMR, or electronic medical records, as well. The capabilities of medical practice management software allow users to streamline clinical workflows by being able to act a repository for patient demographics and document management. The software keeps track of medication lists and history, appointments, insurance eligibility, and manages billing and claims. Many medical practice software allows for e-prescribing functionalities that integrate with pharmacy networks as well as inventory management that permits easy forecasting of stock levels so that orders can be placed ahead of time. 2 CUSTOMER SUCCESS Company Score is affected by the following: SCORING METHODOLOGY 1. Number of employees (based on social media and public resources) The FeaturedCustomers.com Customer 2. Vendor momentum based on web traffic and Success score is based on data from our search trends customer success content platform, social 3. Employee satisfaction and engagement presence, as well as additional data aggregated (based on social network ratings) from online sources and social media properties. 4. % traffic increase to your Customer Our ranking engine applies an algorithm to all of References the data collected to calculate the overall 5. Lower Funnel SEO Key Term Rankings Customer Success score. -

Ehr Software Vendor Directory
EHR SOFTWARE VENDOR DIRECTORY 2017 Edition C M CONVERTED MEDIA OVERVIEW CONTACT ADP acquired AdvancedMD, an established provider of EHR solutions for private practice healthcare environments in 2011. ADP AdvancedMD With ADP, the AdvancedMD product offerings have expanded to include an enhanced in-house billing suite, medical coding 10876 S River Front Pkwy capabilities and integration with outsourced medical billing providers. Suite 400 South Jordan ADP AdvancedMD EHR provides functionality for medical practices as well as components designed for administrative and UT billing use. 84095 United States For medical practices, ADP AdvancedMD EHR provides a patient portal for admissions and check-ins alongside document 800-825-0224 management, e-prescriptions and more. All of this is available as a mobile platform on iOS devices. [email protected] Role-based access to the EHR for nurses and physicians improves workflow by limiting system complexity for each role. During the medical coding and billing phase, there are options to present the information for in-house coders or to integrate it with the API from an outsourced medical billing company. The ADP AdvancedMD EHR and revenue cycle management solutions are available to clients via a tiered subscription access program. There is an initial installation and training fee, and then clients may select “a-la-carte” options for their subscription package that are best suited to their practice. COMPARE ADP ADVANCEDMD EHR & MANY MORE ADP AdvancedMD EHR provides a complete healthcare solution for practices of any size. Their products are cloud based, Compare software features, mobile accessible but can also be installed as desktop only.