The Morphology and Relationships of Dalechampia Scandens (Euphorbiaceae)1
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

CHAPTER 2 REVIEW of the LITERATURE 2.1 Taxa And
CHAPTER 2 REVIEW OF THE LITERATURE 2.1 Taxa and Classification of Acalypha indica Linn., Bridelia retusa (L.) A. Juss. and Cleidion javanicum BL. 2.11 Taxa and Classification of Acalypha indica Linn. Kingdom : Plantae Division : Magnoliophyta Class : Magnoliopsida Order : Euphorbiales Family : Euphorbiaceae Subfamily : Acalyphoideae Genus : Acalypha Species : Acalypha indica Linn. (Saha and Ahmed, 2011) Plant Synonyms: Acalypha ciliata Wall., A. canescens Wall., A. spicata Forsk. (35) Common names: Brennkraut (German), alcalifa (Brazil) and Ricinela (Spanish) (36). 9 2.12 Taxa and Classification of Bridelia retusa (L.) A. Juss. Kingdom : Plantae Division : Magnoliophyta Class : Magnoliopsida Order : Malpighiales Family : Euphorbiaceae Genus : Bridelia Species : Bridelia retusa (L.) A. Juss. Plant Synonyms: Bridelia airy-shawii Li. Common names: Ekdania (37,38). 2.13 Taxa and Classification of Cleidion javanicum BL. Kingdom : Plantae Subkingdom : Tracheobionta Superdivision : Spermatophyta Division : Magnoliophyta Class : Magnoliopsida Subclass : Magnoliopsida Order : Malpighiales Family : Euphorbiaceae Genus : Cleidion Species : Cleidion javanicum BL. Plant Synonyms: Acalypha spiciflora Burm. f. , Lasiostylis salicifolia Presl. Cleidion spiciflorum (Burm.f.) Merr. Common names: Malayalam and Yellari (39). 10 2.2 Review of chemical composition and bioactivities of Acalypha indica Linn., Bridelia retusa (L.) A. Juss. and Cleidion javanicum BL. 2.2.1 Review of chemical composition and bioactivities of Acalypha indica Linn. Acalypha indica -

Mallotus Glomerulatus (Euphorbiaceae Sensu Stricto), a New Species: Description, Pollen and Phylogenetic Position
THAI FOR. BULL. (BOT.) 32: 173–178. 2004. Mallotus glomerulatus (Euphorbiaceae sensu stricto), a new species: description, pollen and phylogenetic position PETER C. VAN WELZEN*, RAYMOND W.J.M. VAN DER HAM*& KRISTO K.M. KULJU* INTRODUCTION A field trip by several staff members of the Forest Herbarium in Bangkok (BKF) to Phu Langka National Park in Nakhon Phanom Province resulted in the discovery of an unusual undershrub up to 1.5 m high and with the typical ‘explosively’ dehiscent fruits of Euphorbiaceae. The two plants showed a unique combination of characters: opposite leaves, stellate hairs, two apical, axillary ‘fruiting columns’ (no real inflorescences), smooth carpels, and a single ovule per locule (typical for the Euphorbiaceae s.s.: subfamilies Acalyphoideae, Crotonoideae, and Euphorbioideae). A year later, other staff members of BKF collected the staminate flowers, which were present in shortly peduncled glomerules. This inflorescence type is quite common in subfamily Phyllanthoideae (now often referred to at the family level as Phyllanthaceae), but all representatives of this (sub)family have two ovules per locule. Thus, the presence of glomerules makes the set of characters unique and we consider the unidentified plant to be a new species. The new species resembles the genus Mallotus in having extrafloral nectaries in the form of round or oval glands on the upper leaf surface, stellate hairs and short, terminal pistillate inflorescences reduced to a single flower. In Thailand the latter character is present in M. calocarpus Airy Shaw. The new species also resembles M. calocarpus in the smooth, unarmed fruits, the penninerved (not triplinerved) leaf blade, short staminate inflorescences (though no glomerules in M. -
![Entry for ACALYPHA Acrogyna Pax [Family EUPHORBIACEAE]](https://docslib.b-cdn.net/cover/8673/entry-for-acalypha-acrogyna-pax-family-euphorbiaceae-78673.webp)
Entry for ACALYPHA Acrogyna Pax [Family EUPHORBIACEAE]
Entry for ACALYPHA acrogyna Pax [family EUPHORBIACEAE] http://plants.jstor.org/flora/flota011327 http://www.jstor.org Your use of the JSTOR archive indicates your acceptance of JSTOR's Terms and Conditions of Use, available at http://www.jstor.org/page/info/about/policies/terms.jsp. JSTOR's Terms and Conditions of Use provides, in part, that unless you have obtained prior permission, you may not download an entire issue of a journal or multiple copies of articles, and you may use content in the JSTOR archive only for your personal, non-commercial use. Please contact the contributing partner regarding any further use of this work. Partner contact information may be obtained at http://plants.jstor.org/page/about/plants/PlantsProject.jsp. Each copy of any part of a JSTOR transmission must contain the same copyright notice that appears on the screen or printed page of such transmission. JSTOR is a not-for-profit service that helps scholars, researchers, and students discover, use, and build upon a wide range of content in a trusted digital archive. We use information technology and tools to increase productivity and facilitate new forms of scholarship. For more information about JSTOR, please contact [email protected]. Page 1 of 2 Entry for ACALYPHA acrogyna Pax [family EUPHORBIACEAE] Herbarium Royal Botanic Gardens, Kew (K) Collection Flora of Tropical Africa Resource Type Reference Sources Entry from Flora of Tropical Africa, Vol 6 Part 1, page 441 (1913) Author: (By J. G. Baker, with additions by C. H. Wright.) Names ACALYPHA acrogyna Pax [family EUPHORBIACEAE], in Engl. Jahrb. -

An Annotated Checklist of the Angiospermic Flora of Rajkandi Reserve Forest of Moulvibazar, Bangladesh
Bangladesh J. Plant Taxon. 25(2): 187-207, 2018 (December) © 2018 Bangladesh Association of Plant Taxonomists AN ANNOTATED CHECKLIST OF THE ANGIOSPERMIC FLORA OF RAJKANDI RESERVE FOREST OF MOULVIBAZAR, BANGLADESH 1 2 A.K.M. KAMRUL HAQUE , SALEH AHAMMAD KHAN, SARDER NASIR UDDIN AND SHAYLA SHARMIN SHETU Department of Botany, Jahangirnagar University, Savar, Dhaka 1342, Bangladesh Keywords: Checklist; Angiosperms; Rajkandi Reserve Forest; Moulvibazar. Abstract This study was carried out to provide the baseline data on the composition and distribution of the angiosperms and to assess their current status in Rajkandi Reserve Forest of Moulvibazar, Bangladesh. The study reports a total of 549 angiosperm species belonging to 123 families, 98 (79.67%) of which consisting of 418 species under 316 genera belong to Magnoliopsida (dicotyledons), and the remaining 25 (20.33%) comprising 132 species of 96 genera to Liliopsida (monocotyledons). Rubiaceae with 30 species is recognized as the largest family in Magnoliopsida followed by Euphorbiaceae with 24 and Fabaceae with 22 species; whereas, in Lilliopsida Poaceae with 32 species is found to be the largest family followed by Cyperaceae and Araceae with 17 and 15 species, respectively. Ficus is found to be the largest genus with 12 species followed by Ipomoea, Cyperus and Dioscorea with five species each. Rajkandi Reserve Forest is dominated by the herbs (284 species) followed by trees (130 species), shrubs (125 species), and lianas (10 species). Woodlands are found to be the most common habitat of angiosperms. A total of 387 species growing in this area are found to be economically useful. 25 species listed in Red Data Book of Bangladesh under different threatened categories are found under Lower Risk (LR) category in this study area. -
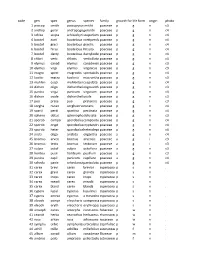
Species List (PDF)
code gen spec genus species family growth formlife form origin photo 1 pascop smith pascopyrumsmithii poaceae p g n c3 2 androp gerar andropogongerardii poaceae p g n c4 3 schiza scopa schizachyriumscoparium poaceae p g n c4 4 boutel curti bouteloua curtipendulapoaceae p g n c4 5 boutel graci bouteloua gracilis poaceae p g n c4 6 boutel hirsu bouteloua hirsuta poaceae p g n c4 7 boutel dacty bouteloua dactyloidespoaceae p g n c4 8 chlori verti chloris verticillata poaceae p g n c4 9 elymus canad elymus canadensispoaceae p g n c3 10 elymus virgi elymus virginicus poaceae p g n c3 11 eragro spect eragrostis spectabilis poaceae p g n c4 12 koeler macra koeleria macrantha poaceae p g n c3 13 muhlen cuspi muhlenbergiacuspidata poaceae p g n c4 14 dichan oligo dichantheliumoligosanthespoaceae p g n c3 15 panicu virga panicum virgatum poaceae p g n c4 16 dichan ovale dichantheliumovale poaceae p g n c3 17 poa prate poa pratensis poaceae p g i c3 18 sorgha nutan sorghastrumnutans poaceae p g n c4 19 sparti pecti spartina pectinata poaceae p g n c4 20 spheno obtus sphenopholisobtusata poaceae p g n c3 21 sporob compo sporoboluscomposituspoaceae p g n c4 22 sporob crypt sporoboluscryptandruspoaceae p g n c4 23 sporob heter sporobolusheterolepispoaceae p g n c4 24 aristi oliga aristida oligantha poaceae a g n c4 25 bromus arven bromus arvensis poaceae a g i c3 26 bromus tecto bromus tectorum poaceae a g i c3 27 vulpia octof vulpia octoflora poaceae a g n c3 28 hordeu pusil hordeum pusillum poaceae a g n c3 29 panicu capil panicum capillare poaceae a g n c4 30 schedo panic schedonnarduspaniculatuspoaceae p g n c4 31 carex brevi carex brevior cyperaceaep s n . -

A Compilation and Analysis of Food Plants Utilization of Sri Lankan Butterfly Larvae (Papilionoidea)
MAJOR ARTICLE TAPROBANICA, ISSN 1800–427X. August, 2014. Vol. 06, No. 02: pp. 110–131, pls. 12, 13. © Research Center for Climate Change, University of Indonesia, Depok, Indonesia & Taprobanica Private Limited, Homagama, Sri Lanka http://www.sljol.info/index.php/tapro A COMPILATION AND ANALYSIS OF FOOD PLANTS UTILIZATION OF SRI LANKAN BUTTERFLY LARVAE (PAPILIONOIDEA) Section Editors: Jeffrey Miller & James L. Reveal Submitted: 08 Dec. 2013, Accepted: 15 Mar. 2014 H. D. Jayasinghe1,2, S. S. Rajapaksha1, C. de Alwis1 1Butterfly Conservation Society of Sri Lanka, 762/A, Yatihena, Malwana, Sri Lanka 2 E-mail: [email protected] Abstract Larval food plants (LFPs) of Sri Lankan butterflies are poorly documented in the historical literature and there is a great need to identify LFPs in conservation perspectives. Therefore, the current study was designed and carried out during the past decade. A list of LFPs for 207 butterfly species (Super family Papilionoidea) of Sri Lanka is presented based on local studies and includes 785 plant-butterfly combinations and 480 plant species. Many of these combinations are reported for the first time in Sri Lanka. The impact of introducing new plants on the dynamics of abundance and distribution of butterflies, the possibility of butterflies being pests on crops, and observations of LFPs of rare butterfly species, are discussed. This information is crucial for the conservation management of the butterfly fauna in Sri Lanka. Key words: conservation, crops, larval food plants (LFPs), pests, plant-butterfly combination. Introduction Butterflies go through complete metamorphosis 1949). As all herbivorous insects show some and have two stages of food consumtion. -

Euphorbia Telephioides (Euphorbiaceae)
Genetic diversity within a threatened, endemic North American species, Euphorbia telephioides (Euphorbiaceae) Dorset W. Trapnell, J. L. Hamrick & Vivian Negrón-Ortiz Conservation Genetics ISSN 1566-0621 Conserv Genet DOI 10.1007/s10592-012-0323-4 1 23 Your article is protected by copyright and all rights are held exclusively by Springer Science+Business Media B.V.. This e-offprint is for personal use only and shall not be self- archived in electronic repositories. If you wish to self-archive your work, please use the accepted author’s version for posting to your own website or your institution’s repository. You may further deposit the accepted author’s version on a funder’s repository at a funder’s request, provided it is not made publicly available until 12 months after publication. 1 23 Author's personal copy Conserv Genet DOI 10.1007/s10592-012-0323-4 RESEARCH ARTICLE Genetic diversity within a threatened, endemic North American species, Euphorbia telephioides (Euphorbiaceae) Dorset W. Trapnell • J. L. Hamrick • Vivian Negro´n-Ortiz Received: 23 September 2011 / Accepted: 20 January 2012 Ó Springer Science+Business Media B.V. 2012 Abstract The southeastern United States and Florida which it occurs, Gulf (0.084), Franklin (0.059) and Bay support an unusually large number of endemic plant spe- Counties (0.033), were also quite low. Peripheral popula- cies, many of which are threatened by anthropogenic tions did not generally have reduced genetic variation habitat disturbance. As conservation measures are under- although there was significant isolation by distance. Rare- taken and recovery plans designed, a factor that must be faction analysis showed a non-significant relationship taken into consideration is the genetic composition of the between allelic richness and actual population sizes. -

Euphorbia Subg
ФЕДЕРАЛЬНОЕ ГОСУДАРСТВЕННОЕ БЮДЖЕТНОЕ УЧРЕЖДЕНИЕ НАУКИ БОТАНИЧЕСКИЙ ИНСТИТУТ ИМ. В.Л. КОМАРОВА РОССИЙСКОЙ АКАДЕМИИ НАУК На правах рукописи Гельтман Дмитрий Викторович ПОДРОД ESULA РОДА EUPHORBIA (EUPHORBIACEAE): СИСТЕМА, ФИЛОГЕНИЯ, ГЕОГРАФИЧЕСКИЙ АНАЛИЗ 03.02.01 — ботаника ДИССЕРТАЦИЯ на соискание ученой степени доктора биологических наук САНКТ-ПЕТЕРБУРГ 2015 2 Оглавление Введение ......................................................................................................................................... 3 Глава 1. Род Euphorbia и основные проблемы его систематики ......................................... 9 1.1. Общая характеристика и систематическое положение .......................................... 9 1.2. Краткая история таксономического изучения и формирования системы рода ... 10 1.3. Основные проблемы систематики рода Euphorbia и его подрода Esula на рубеже XX–XXI вв. и пути их решения ..................................................................................... 15 Глава 2. Материал и методы исследования ........................................................................... 17 Глава 3. Построение системы подрода Esula рода Euphorbia на основе молекулярно- филогенетического подхода ...................................................................................................... 24 3.1. Краткая история молекулярно-филогенетического изучения рода Euphorbia и его подрода Esula ......................................................................................................... 24 3.2. Результаты молекулярно-филогенетического -

Gender Variation in CROTON CALIFORNICUS (EUPHORBIACEAE)
Loma Linda University TheScholarsRepository@LLU: Digital Archive of Research, Scholarship & Creative Works Loma Linda University Electronic Theses, Dissertations & Projects 9-1999 Gender Variation in CROTON CALIFORNICUS (EUPHORBIACEAE) James Lynwood Smith II Follow this and additional works at: https://scholarsrepository.llu.edu/etd Part of the Biology Commons, and the Botany Commons Recommended Citation Smith, James Lynwood II, "Gender Variation in CROTON CALIFORNICUS (EUPHORBIACEAE)" (1999). Loma Linda University Electronic Theses, Dissertations & Projects. 946. https://scholarsrepository.llu.edu/etd/946 This Dissertation is brought to you for free and open access by TheScholarsRepository@LLU: Digital Archive of Research, Scholarship & Creative Works. It has been accepted for inclusion in Loma Linda University Electronic Theses, Dissertations & Projects by an authorized administrator of TheScholarsRepository@LLU: Digital Archive of Research, Scholarship & Creative Works. For more information, please contact [email protected]. LOMA LINDA UNIVERSITY Graduate School GENDER VARIATION IN CROTON CALIFORNICUS (EUPHORBIACEAE) by James Lynwood Smith II A Dissertation in Partial Fulfillment of the Requirements for the Degree Doctor of Philosophy in Biology September 1999 Each person whose signature appears below certifies that this dissertation in their opinion is adequate, in scope and quality, as a dissertation for the degree of Doctor of Philosophy. , Co-Chairperson , Co-Chairperson Gary L. Bradley, Professor at La Sierra University ii Acknowledgments I wish to thank Ron Carter and Brad Martin for their guidance, assistance, and comments. I am grateful to the other members of my guidance committee, Gary Bradley, Bob Cushman, and Bill Hayes, for their advice and comments. I am also grateful to Aida Smith for assisting with data collection, data entry, and providing comments. -

Dalechampia COM ÊNFASE EM Dalechampia Sect
RAFAELA ALVES PEREIRA DA SILVA FILOGENIA E TAXONOMIA DE Dalechampia COM ÊNFASE EM Dalechampia sect. Dalechampia, Euphorbiaceae RECIFE 2019 I RAFAELA ALVES PEREIRA DA SILVA FILOGENIA E TAXONOMIA DE Dalechampia COM ÊNFASE EM Dalechampia sect. Dalechampia, Euphorbiaceae Tese apresentada ao Programa de Pós-Graduação em Botânica da Universidade Federal Rural de Pernambuco, como parte dos requisitos para obtenção do título de Doutora em Botânica. Orientadora: Profª. Dra. Margareth Ferreira de Sales Deptº de Biologia, Área de Botânica/UFRPE Co-orientadores: Dra. Sarah Maria Athiê-Souza Profº. Dr. Luís Gustavo Rodrigues de Souza Colaborador: Dr. Scott Armbruster Profº. Dr. André Laurênio de Melo RECIFE 2019 II Dados Internacionais de Catalogação na Publicação (CIP) Sistema Integrado de Bibliotecas da UFRPE Biblioteca Central, Recife-PE, Brasil S586f Silva, Rafaela Alves Pereira da. Filogenia e taxonomia de Dalechampia com ênfase em Dalechampia sect. Dalechampia, Euphorbiaceae / Rafaela Alves Pereira da Silva. – Recife, 2019. 335 f.: il. Orientador(a): Margareth Ferreira de Sales. Coorientador(a): Sarah Maria Athiê-Souza Coorientador(a): Luís Gustavo Rodrigues de Souza Tese (Doutorado) – Universidade Federal Rural de Pernambuco, Programa de Pós-Graduação em Botânica, Recife, BR-PE, 2019. Inclui referências e apêndice(s). 1. Dalechampiinae 2. Biogeography 3. Molecular 4. Character evolution I. Sales, Margareth Ferreira de, orient. II. Athiê-Souza, Sarah Maria, coorient. III Souza, Luís Gustavo Rodrigues de, coorient. IV. Título CDD 581 III FILOGENIA E TAXONOMIA DE Dalechampia COM ÊNFASE EM Dalechampia sect. Dalechampia, Euphorbiaceae IV Dedico Ao Espírito Santo de Deus. Ofereço A Ednaldo José da Silva “Ignore aquele que diz: você não tem valor por isso ou por aquilo, porque eu te amo muito e torço por você”. -

Recovery Plan for Tyoj5llllt . I-Bland Plants
Recovery Plan for tYOJ5llllt. i-bland Plants RECOVERY PLAN FOR MULTI-ISLAND PLANTS Published by U.S. Fish and Wildlife Service Portland, Oregon Approved: Date: / / As the Nation’s principal conservation agency, the Department of the Interior has responsibility for most ofour nationally owned public lands and natural resources. This includes fostering the wisest use ofour land and water resources, protecting our fish and wildlife, preserving the environmental and cultural values ofour national parks and historical places, and providing for the enjoyment of life through outdoor recreation. The Department assesses our energy and mineral resources and works to assure that their development is in the best interests ofall our people. The Department also has a major responsibility for American Indian reservation communities and for people who live in island Territories under U.S. administration. DISCLAIMER PAGE Recovery plans delineate reasonable actions that are believed to be required to recover and/or protect listed species. Plans are published by the U.S. Fish and Wildlife Service, sometimes prepared with the assistance ofrecovery teams, contractors, State agencies, and others. Objectives will be attained and any necessary funds made available subject to budgetary and other constraints affecting the parties involved, as well as the need to address other priorities. Costs indicated for task implementation and/or time for achievement ofrecovery are only estimates and are subject to change. Recovery plans do not necessarily represent the views nor the official positions or approval ofany individuals or agencies involved in the plan formulation, otherthan the U.S. Fish and Wildlife Service. They represent the official position ofthe U.S. -

9. Herbs and Its Amazing Healing Properties
EPTRI‐ENVIS Centre (Ecology of Eastern Ghats) HERBS AND ITS AMAZING HEALING PROPERTIES Article 04/2015/ENVIS-Ecology of Eastern Ghats Page 1 of 50 EPTRI‐ENVIS Centre (Ecology of Eastern Ghats) LIST OF MEDICINAL HERBS Plant name : Achyranthes aspera L. Family : Amaranthaceae Local name : Uttareni Habit : Herb Fl & Fr time : October – March Part(s) used : Leaves Medicinal uses : Leaf paste is applied externally for eye pain and dog bite. Internally taken leaves decoction with water/milk to cure stomach problems, diuretic, piles and skin diseases. Plant name : Abelmoschus esculentus (L.) Moench. Family : Malvaceae Local name : Benda Habit : Herb Fl & Fr time : Part(s) used : Roots Medicinal uses : The juice of the roots is used externally to treat cuts, wounds and boils. Plant name : Abutilon crispum (L.) Don Family : Malvaceae Local name : Nelabenda Habit : Herb Fl & Fr time : March – September Part(s) used : Root Medicinal uses : Root is used for the treatment of nervous disorders. Article 04/2015/ENVIS-Ecology of Eastern Ghats Page 2 of 50 EPTRI‐ENVIS Centre (Ecology of Eastern Ghats) Plant name : Abutilon indicum (L.) Sweet Family : Malvaceae Local name : Thuttutubenda Habit : Herb Fl & Fr time : March – September Part(s) used : Leaves & Roots Medicinal uses : Leaf juice is used for the treatment of toothache. Roots and leaves decoction is given for diuretic and stimulate purgative. Plant name : Abrus precatorius L. Family : Fabaceae Local name : Guruvenda Habit : Herb Fl & Fr time : July – December Part(s) used : Root & Seeds Medicinal uses : Roots used to treat poisonous bite and seed is used to treat leucoderma Plant name : Acalypha indica L.