Chapter 2 the International System of Measuring Units (SI)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Appendix A: Symbols and Prefixes
Appendix A: Symbols and Prefixes (Appendix A last revised November 2020) This appendix of the Author's Kit provides recommendations on prefixes, unit symbols and abbreviations, and factors for conversion into units of the International System. Prefixes Recommended prefixes indicating decimal multiples or submultiples of units and their symbols are as follows: Multiple Prefix Abbreviation 1024 yotta Y 1021 zetta Z 1018 exa E 1015 peta P 1012 tera T 109 giga G 106 mega M 103 kilo k 102 hecto h 10 deka da 10-1 deci d 10-2 centi c 10-3 milli m 10-6 micro μ 10-9 nano n 10-12 pico p 10-15 femto f 10-18 atto a 10-21 zepto z 10-24 yocto y Avoid using compound prefixes, such as micromicro for pico and kilomega for giga. The abbreviation of a prefix is considered to be combined with the abbreviation/symbol to which it is directly attached, forming with it a new unit symbol, which can be raised to a positive or negative power and which can be combined with other unit abbreviations/symbols to form abbreviations/symbols for compound units. For example: 1 cm3 = (10-2 m)3 = 10-6 m3 1 μs-1 = (10-6 s)-1 = 106 s-1 1 mm2/s = (10-3 m)2/s = 10-6 m2/s Abbreviations and Symbols Whenever possible, avoid using abbreviations and symbols in paragraph text; however, when it is deemed necessary to use such, define all but the most common at first use. The following is a recommended list of abbreviations/symbols for some important units. -

Foot-Candles: Photometric Units
UPDATED EXTRACT FROM CREG JOURNAL. FILE: FOOT3-UP.DOC REV. 8. LAST SAVED: 01/02/01 15:14 PHOTOMETRICS Foot-Candles: Photometric Units More footnotes on optical topics. David Gibson describes the confusing range of photometric units. A discussion of photometric units may the ratio of luminous efficiency to luminous The non-SI unit mean spherical candle- seem out of place in an electronic journal but efficiency at the wavelength where the eye is power is the intensity of a source if its light engineers frequently have to use light sources most sensitive. Unfortunately, however, this output were spread evenly in all directions. It and detectors. The units of photometry are term can be confused with the term efficacy, is therefore equivalent to the flux [lm] ¸ 4p. some of the most confusing and least which is used to describe the efficiency at standardised of units. converting electrical to luminous power. Luminance Photometric units are not difficult to The candela measures the intensity of a understand, but can be a minefield to the Illumination, Luminous Emittance. point source. We also need to define the uninitiated since many non-SI units are still The illumination of a surface is the properties of an extended source. Each small in use, and there are subtle differences incident power flux density measured in element DS of a diffuse reflective surface will between quantities with similar names, such lumens per square metre. A formal definition scatter the incident flux DF and behave as if as illumination and luminance. would be along the lines of: if a flux DF is it were an infinitesimal point source. -

A Review of Energy Storage Technologies' Application
sustainability Review A Review of Energy Storage Technologies’ Application Potentials in Renewable Energy Sources Grid Integration Henok Ayele Behabtu 1,2,* , Maarten Messagie 1, Thierry Coosemans 1, Maitane Berecibar 1, Kinde Anlay Fante 2 , Abraham Alem Kebede 1,2 and Joeri Van Mierlo 1 1 Mobility, Logistics, and Automotive Technology Research Centre, Vrije Universiteit Brussels, Pleinlaan 2, 1050 Brussels, Belgium; [email protected] (M.M.); [email protected] (T.C.); [email protected] (M.B.); [email protected] (A.A.K.); [email protected] (J.V.M.) 2 Faculty of Electrical and Computer Engineering, Jimma Institute of Technology, Jimma University, Jimma P.O. Box 378, Ethiopia; [email protected] * Correspondence: [email protected]; Tel.: +32-485659951 or +251-926434658 Received: 12 November 2020; Accepted: 11 December 2020; Published: 15 December 2020 Abstract: Renewable energy sources (RESs) such as wind and solar are frequently hit by fluctuations due to, for example, insufficient wind or sunshine. Energy storage technologies (ESTs) mitigate the problem by storing excess energy generated and then making it accessible on demand. While there are various EST studies, the literature remains isolated and dated. The comparison of the characteristics of ESTs and their potential applications is also short. This paper fills this gap. Using selected criteria, it identifies key ESTs and provides an updated review of the literature on ESTs and their application potential to the renewable energy sector. The critical review shows a high potential application for Li-ion batteries and most fit to mitigate the fluctuation of RESs in utility grid integration sector. -

Guide for the Use of the International System of Units (SI)
Guide for the Use of the International System of Units (SI) m kg s cd SI mol K A NIST Special Publication 811 2008 Edition Ambler Thompson and Barry N. Taylor NIST Special Publication 811 2008 Edition Guide for the Use of the International System of Units (SI) Ambler Thompson Technology Services and Barry N. Taylor Physics Laboratory National Institute of Standards and Technology Gaithersburg, MD 20899 (Supersedes NIST Special Publication 811, 1995 Edition, April 1995) March 2008 U.S. Department of Commerce Carlos M. Gutierrez, Secretary National Institute of Standards and Technology James M. Turner, Acting Director National Institute of Standards and Technology Special Publication 811, 2008 Edition (Supersedes NIST Special Publication 811, April 1995 Edition) Natl. Inst. Stand. Technol. Spec. Publ. 811, 2008 Ed., 85 pages (March 2008; 2nd printing November 2008) CODEN: NSPUE3 Note on 2nd printing: This 2nd printing dated November 2008 of NIST SP811 corrects a number of minor typographical errors present in the 1st printing dated March 2008. Guide for the Use of the International System of Units (SI) Preface The International System of Units, universally abbreviated SI (from the French Le Système International d’Unités), is the modern metric system of measurement. Long the dominant measurement system used in science, the SI is becoming the dominant measurement system used in international commerce. The Omnibus Trade and Competitiveness Act of August 1988 [Public Law (PL) 100-418] changed the name of the National Bureau of Standards (NBS) to the National Institute of Standards and Technology (NIST) and gave to NIST the added task of helping U.S. -

An Evaluation of Turbocharging and Supercharging Options for High-Efficiency Fuel Cell Electric Vehicles
applied sciences Article An Evaluation of Turbocharging and Supercharging Options for High-Efficiency Fuel Cell Electric Vehicles Arthur Kerviel 1,2, Apostolos Pesyridis 1,* , Ahmed Mohammed 1 and David Chalet 2 1 Department of Mechanical and Aerospace Engineering, Brunel University, London UB8 3PH, UK; [email protected] (A.K.); [email protected] (A.M.) 2 Ecole Centrale de Nantes, LHEEA Lab. (ECN/CNRS), 44321 Nantes, France; [email protected] * Correspondence: [email protected]; Tel.: +44-189-526-7901 Received: 17 October 2018; Accepted: 19 November 2018; Published: 3 December 2018 Abstract: Mass-produced, off-the-shelf automotive air compressors cannot be directly used for boosting a fuel cell vehicle (FCV) application in the same way that they are used in internal combustion engines, since the requirements are different. These include a high pressure ratio, a low mass flow rate, a high efficiency requirement, and a compact size. From the established fuel cell types, the most promising for application in passenger cars or light commercial vehicle applications is the proton exchange membrane fuel cell (PEMFC), operating at around 80 ◦C. In this case, an electric-assisted turbocharger (E-turbocharger) and electric supercharger (single or two-stage) are more suitable than screw and scroll compressors. In order to determine which type of these boosting options is the most suitable for FCV application and assess their individual merits, a co-simulation of FCV powertrains between GT-SUITE and MATLAB/SIMULINK is realised to compare vehicle performance on the Worldwide Harmonised Light Vehicle Test Procedure (WLTP) driving cycle. -

Da Ferraris a Giorgi: La Scuola Italiana Di Elettrotecnica
Da Ferraris a Giorgi: la scuola italiana di Elettrotecnica ADRIANO PAOLO MORANDO Da Ferraris a Giorgi: la scuola italiana di Elettrotecnica Implicita nella Dynamical collocano in modo preciso, facendone Philosophy e nella espansione di una il continuatore, nel solco dell’opera rivoluzione industriale in atto, la nasci- ferrarisiana. Il primo, The foundations ta dell’ingegneria elettrica scientifica si of Electrical Science, del 1894, riporta articolò, postmaxwellianamente, nella a quell’approccio operativo di progressiva transizione dalla figura del- Bridgman che avrebbe in seguito in- lo scienziato (Maxwell), a quella dello fluenzato la lettura di Ercole Bottani. Il scienziato-inventore (Ferraris), a quel- secondo, del 1905, è legato alla Teoria la, infine, del fisico matematico che di- della dinamo ricorsiva, con la quale venta ingegnere (Steinmetz). egli, sulla scia di alcuni contributi Sottolineato lo stretto legame che, ferrarisiani ed anticipando la trasformata sul piano metodologico e fondazionale, di Park, propose un primo approccio correlò la Dynamical Theory maxwel- elettrodinamico alla teoria unificata del- liana ai contributi recati da Galileo le macchine elettriche. Ferraris alla teoria scientifica del trasfor- Prendendo le mosse da questi ele- matore e all’invenzione del campo ro- menti, verranno ravvisati, lungo il per- tante, si colloca, nel quadro della cultu- corso Mossotti, Codazza, Ferraris, ra e della società del tempo, la figura Ascoli-Arnò e Giorgi-Lori, gli elemen- dello scienziato italiano. Ne emerge, tra- ti portanti della scuola italiana di mite il suo maestro Giovanni Codazza, elettrotecnica. il ruolo decisivo che sulla sua forma- zione ebbero - oltre a Maxwell, Tait, von L’astuzia scozzese: la dynamical Helmholtz ed Heaviside - il maestro di philosophy questi: Fabrizio Ottaviano Mossotti. -

Ces-Collins-Pp-Ev-Conundrum
Gabriel Collins, J.D. Baker Botts Fellow for Energy & Environmental Regulatory Affairs Baker Institute for Public Policy, Rice University EVs = Power-to-WeigHt Ratio of an Aircraft, Onboard Fuel of a Subcompact Car • Electric veHicles’ instant torque and higH horsepower make the higher-end editions true performance monsters. Their power-to-weigHt ratios are often on par witH turboprop aircraft and many helicopters. • The fly in the ointment comes from the fact that batteries remain heavy and relative to hydrocarbons and simply cannot store energy nearly as efficiently per unit of mass. • The power-to-weigHt/onboard energy density relationsHip of the Rivian R1T electric truck and A-29 Big, long-range Super Tucano attack aircraft is sucH that platforms “Rivianizing” the Tucano would mean making its onboard jet fuel stores retain their current energy Super Tucano (17) and Rivian R1T (14) content, but weigH more per liter than lead metal. Needless to say, the plane’s fligHt endurance and carrying capacity would fall dramatically. • This estimate assumes the A-29’s PT-6 turboprop has a thermal efficiency of about 35%--less than half the R1T’s likely capacity to convert fuel into actual propulsive energy. Please cite as: Gabriel Collins, “The EV Conundrum: High Power Density and Low Energy Density,” Baker Institute ResearcH Source: Beechcraft, Car & Driver, EIA, EV Database, GE, Global Security, Man, Nikola, Peterbuilt, Rivian, SNC, Trucks.com, USAF, US Navy Presentation, 8 January 2020, Houston, TX Gabriel Collins, J.D. Baker Botts Fellow for Energy & Environmental Regulatory Affairs Baker Institute for Public Policy, Rice University Electric Vehicles’ Onboard Energy Still Significantly Trails ICE Vehicles On an Efficiency-Adjusted Basis Efficiency-Adjusted Comparison • To make the energy density comparison a bit more fair, we adjust the onboard fuel based on the fact that EVs convert nearly 80% of their battery energy into tractive power at the wheels, while IC vehicles feature efficiencies closer to 20%. -

SI and CGS Units in Electromagnetism
SI and CGS Units in Electromagnetism Jim Napolitano January 7, 2010 These notes are meant to accompany the course Electromagnetic Theory for the Spring 2010 term at RPI. The course will use CGS units, as does our textbook Classical Electro- dynamics, 2nd Ed. by Hans Ohanian. Up to this point, however, most students have used the International System of Units (SI, also known as MKSA) for mechanics, electricity and magnetism. (I believe it is easy to argue that CGS is more appropriate for teaching elec- tromagnetism to physics students.) These notes are meant to smooth the transition, and to augment the discussion in Appendix 2 of your textbook. The base units1 for mechanics in SI are the meter, kilogram, and second (i.e. \MKS") whereas in CGS they are the centimeter, gram, and second. Conversion between these base units and all the derived units are quite simply given by an appropriate power of 10. For electromagnetism, SI adds a new base unit, the Ampere (\A"). This leads to a world of complications when converting between SI and CGS. Many of these complications are philosophical, but this note does not discuss such issues. For a good, if a bit flippant, on- line discussion see http://info.ee.surrey.ac.uk/Workshop/advice/coils/unit systems/; for a more scholarly article, see \On Electric and Magnetic Units and Dimensions", by R. T. Birge, The American Physics Teacher, 2(1934)41. Electromagnetism: A Preview Electricity and magnetism are not separate phenomena. They are different manifestations of the same phenomenon, called electromagnetism. One requires the application of special relativity to see how electricity and magnetism are united. -

Photography Department Cerritos College
Program Review 2010 Photography Department Cerritos College 1. Description of the Program Mission Statement The mission of the photography department is to educate students in photography by providing a balanced education in both the creative and technical aspects of the photographic medium, the history of photography and the critical analysis of photographs. The mission is accomplished through course offerings that enable students to develop their technical skills, creativity and understanding of the social and historical role and relevance of the photographic medium. a. Course and Program Content i. Course Outlines and Curriculum Development Process The following Photography Department courses have been approved by the curriculum committee: Phot 100 - Introductory Photography (revised and approved 1/25/07) Phot 102 - Intermediate Photography (revised and approved 1/27/05) Phot 110 - Introduction to Digital Photography (revised and approved 3/10/05) Phot 112 - Intermediate Digital Photography (revised and approved 3/10/05) Phot 122 - Color Photography (revised and approved 10/26/06) Phot 130 - Jour 130 - Photojournalism (revised and approved 12/13/07) Phot 150 - Photography and Society (approved 2/26/09) Phot 160 - History of Photography (revised and approved 1/25/07) Phot 171 - Occupational Work Experience in Photography (revised and approved 9/22/05) Phot 200 - Advanced Photography (revised and approved 1/27/05) Phot 230 - Portrait Photography (revised and approved 1/27/05) Phot 232 - Approaches to Studio Lighting (revised and approved 1/27/05) Phot 234 - Portfolio Development (revised and approved 1/26/06) Phot 240 - Experimental Photography (revised and approved 2/24/05) Phot 250 - Contemporary Photographic Expression (revised and approved 12/11/03) Phot 252 - Photography of the Landscape (revised and approved 1/27/05) Phot 298 - Directed Studies Phot 299 - Directed Studies The only course in the list above that has not yet been offered is Photography and Society. -

DIMENSIONS and UNITS to Get the Value of a Quantity in Gaussian Units, Multiply the Value Ex- Pressed in SI Units by the Conversion Factor
DIMENSIONS AND UNITS To get the value of a quantity in Gaussian units, multiply the value ex- pressed in SI units by the conversion factor. Multiples of 3 intheconversion factors result from approximating the speed of light c =2.9979 1010 cm/sec × 3 1010 cm/sec. ≈ × Dimensions Physical Sym- SI Conversion Gaussian Quantity bol SI Gaussian Units Factor Units t2q2 Capacitance C l farad 9 1011 cm ml2 × m1/2l3/2 Charge q q coulomb 3 109 statcoulomb t × q m1/2 Charge ρ coulomb 3 103 statcoulomb 3 3/2 density l l t /m3 × /cm3 tq2 l Conductance siemens 9 1011 cm/sec ml2 t × 2 tq 1 9 1 Conductivity σ siemens 9 10 sec− 3 ml t /m × q m1/2l3/2 Current I,i ampere 3 109 statampere t t2 × q m1/2 Current J, j ampere 3 105 statampere 2 1/2 2 density l t l t /m2 × /cm2 m m 3 3 3 Density ρ kg/m 10− g/cm l3 l3 q m1/2 Displacement D coulomb 12π 105 statcoulomb l2 l1/2t /m2 × /cm2 1/2 ml m 1 4 Electric field E volt/m 10− statvolt/cm t2q l1/2t 3 × 2 1/2 1/2 ml m l 1 2 Electro- , volt 10− statvolt 2 motance EmfE t q t 3 × ml2 ml2 Energy U, W joule 107 erg t2 t2 m m Energy w, ϵ joule/m3 10 erg/cm3 2 2 density lt lt 10 Dimensions Physical Sym- SI Conversion Gaussian Quantity bol SI Gaussian Units Factor Units ml ml Force F newton 105 dyne t2 t2 1 1 Frequency f, ν hertz 1 hertz t t 2 ml t 1 11 Impedance Z ohm 10− sec/cm tq2 l 9 × 2 2 ml t 1 11 2 Inductance L henry 10− sec /cm q2 l 9 × Length l l l meter (m) 102 centimeter (cm) 1/2 q m 3 Magnetic H ampere– 4π 10− oersted 1/2 intensity lt l t turn/m × ml2 m1/2l3/2 Magnetic flux Φ weber 108 maxwell tq t m m1/2 Magnetic -
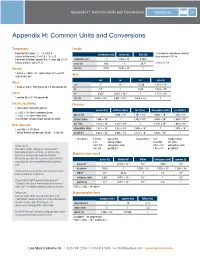
Appendix H: Common Units and Conversions Appendices 215
Appendix H: Common Units and Conversions Appendices 215 Appendix H: Common Units and Conversions Temperature Length Fahrenheit to Celsius: °C = (°F-32)/1.8 1 micrometer (sometimes referred centimeter (cm) meter (m) inch (in) Celsius to Fahrenheit: °F = (1.8 × °C) + 32 to as micron) = 10-6 m Fahrenheit to Kelvin: convert °F to °C, then add 273.15 centimeter (cm) 1 1.000 × 10–2 0.3937 -3 Celsius to Kelvin: add 273.15 meter (m) 100 1 39.37 1 mil = 10 in Volume inch (in) 2.540 2.540 × 10–2 1 –3 3 1 liter (l) = 1.000 × 10 cubic meters (m ) = 61.02 Area cubic inches (in3) cm2 m2 in2 circ mil Mass cm2 1 10–4 0.1550 1.974 × 105 1 kilogram (kg) = 1000 grams (g) = 2.205 pounds (lb) m2 104 1 1550 1.974 × 109 Force in2 6.452 6.452 × 10–4 1 1.273 × 106 1 newton (N) = 0.2248 pounds (lb) circ mil 5.067 × 10–6 5.067 × 10–10 7.854 × 10–7 1 Electric resistivity Pressure 1 micro-ohm-centimeter (µΩ·cm) pascal (Pa) millibar (mbar) torr (Torr) atmosphere (atm) psi (lbf/in2) = 1.000 × 10–6 ohm-centimeter (Ω·cm) –2 –3 –6 –4 = 1.000 × 10–8 ohm-meter (Ω·m) pascal (Pa) 1 1.000 × 10 7.501 × 10 9.868 × 10 1.450 × 10 = 6.015 ohm-circular mil per foot (Ω·circ mil/ft) millibar (mbar) 1.000 × 102 1 7.502 × 10–1 9.868 × 10–4 1.450 × 10–2 2 0 –3 –2 Heat flow rate torr (Torr) 1.333 × 10 1.333 × 10 1 1.316 × 10 1.934 × 10 5 3 2 1 1 watt (W) = 3.413 Btu/h atmosphere (atm) 1.013 × 10 1.013 × 10 7.600 × 10 1 1.470 × 10 1 British thermal unit per hour (Btu/h) = 0.2930 W psi (lbf/in2) 6.897 × 103 6.895 × 101 5.172 × 101 6.850 × 10–2 1 1 torr (Torr) = 133.332 pascal (Pa) 1 pascal (Pa) = 0.01 millibar (mbar) 1.33 millibar (mbar) 0.007501 torr (Torr) –6 A Note on SI 0.001316 atmosphere (atm) 9.87 × 10 atmosphere (atm) 2 –4 2 The values in this catalog are expressed in 0.01934 psi (lbf/in ) 1.45 × 10 psi (lbf/in ) International System of Units, or SI (from the Magnetic induction B French Le Système International d’Unités). -

The International System of Units (SI)
NAT'L INST. OF STAND & TECH NIST National Institute of Standards and Technology Technology Administration, U.S. Department of Commerce NIST Special Publication 330 2001 Edition The International System of Units (SI) 4. Barry N. Taylor, Editor r A o o L57 330 2oOI rhe National Institute of Standards and Technology was established in 1988 by Congress to "assist industry in the development of technology . needed to improve product quality, to modernize manufacturing processes, to ensure product reliability . and to facilitate rapid commercialization ... of products based on new scientific discoveries." NIST, originally founded as the National Bureau of Standards in 1901, works to strengthen U.S. industry's competitiveness; advance science and engineering; and improve public health, safety, and the environment. One of the agency's basic functions is to develop, maintain, and retain custody of the national standards of measurement, and provide the means and methods for comparing standards used in science, engineering, manufacturing, commerce, industry, and education with the standards adopted or recognized by the Federal Government. As an agency of the U.S. Commerce Department's Technology Administration, NIST conducts basic and applied research in the physical sciences and engineering, and develops measurement techniques, test methods, standards, and related services. The Institute does generic and precompetitive work on new and advanced technologies. NIST's research facilities are located at Gaithersburg, MD 20899, and at Boulder, CO 80303.