2005 Nobel Laureates
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

RANDY SCHEKMAN Department of Molecular and Cell Biology, Howard Hughes Medical Institute, University of California, Berkeley, USA
GENES AND PROTEINS THAT CONTROL THE SECRETORY PATHWAY Nobel Lecture, 7 December 2013 by RANDY SCHEKMAN Department of Molecular and Cell Biology, Howard Hughes Medical Institute, University of California, Berkeley, USA. Introduction George Palade shared the 1974 Nobel Prize with Albert Claude and Christian de Duve for their pioneering work in the characterization of organelles interrelated by the process of secretion in mammalian cells and tissues. These three scholars established the modern field of cell biology and the tools of cell fractionation and thin section transmission electron microscopy. It was Palade’s genius in particular that revealed the organization of the secretory pathway. He discovered the ribosome and showed that it was poised on the surface of the endoplasmic reticulum (ER) where it engaged in the vectorial translocation of newly synthesized secretory polypeptides (1). And in a most elegant and technically challenging investigation, his group employed radioactive amino acids in a pulse-chase regimen to show by autoradiograpic exposure of thin sections on a photographic emulsion that secretory proteins progress in sequence from the ER through the Golgi apparatus into secretory granules, which then discharge their cargo by membrane fusion at the cell surface (1). He documented the role of vesicles as carriers of cargo between compartments and he formulated the hypothesis that membranes template their own production rather than form by a process of de novo biogenesis (1). As a university student I was ignorant of the important developments in cell biology; however, I learned of Palade’s work during my first year of graduate school in the Stanford biochemistry department. -

Francois Jacob Memorial
RETROSPECTIVE RETROSPECTIVE Francois Jacob memorial Arthur B. Pardee1 Department of Adult Oncology, Dana-Farber Institute, Boston, MA 02115 Dr. Francois Jacob is one of a handful of the DNA would integrate into the bacterial chro- 20th century’smostdistinguishedlifescien- mosome and remain dormant or, at other tists. His research with Dr. Jacques Monod, times, would kill the cell. like that of Watson and Crick, provided the Jacob’s next major contribution, in collab- foundations for understanding mechanisms oration with Dr. Jacques Monod, was to in- of genetic regulation of life processes such vestigate how a gene is regulated. Remark- as cell differentiation and defects in diseases. ably, native E. coli synthesize β-galactosidase Jacob joined the College de France in 1964 only when lactose is available. Some mutated and shared the Nobel Prize in Physiology bacteria can make the enzyme in the absence or Medicine 1965 with Jacques Monod of inducer. Monod’s initial idea was that and Andre Lwoff. He was elected to the these constitutive bacteria activate the gene National Academy of Sciences (NAS) USA by synthesizing an intracellular lactose-like in 1969. inducer molecule. Jacob was born in 1920 in a French Jewish To investigate this model, interrupted family; his grandfather was a four-star gen- mating was applied to bring the β-galactosi- eral. He began to study medicine before dase gene of a donor bacterium into a consti- World War II, in which he served as a mil- tutive receptor. According to the induction itary officer in the Free French Army and was model, the mated cell should produce en- badly wounded in an air raid. -

INTERNATIONAL INSTITUTE of BENGAL and HIMALAYAN BASINS 10 Evans Hall, University of California at Berkeley Berkeley, California
INTERNATIONAL INSTITUTE OF BENGAL AND HIMALAYAN BASINS 10 Evans Hall, University of California at Berkeley BERKELEY, CALIFORNIA The International Institute of the Bengal and Himalayan Basins PEACE July 20, 2013 TOWNES AND TAGORE FOURTH ANNUAL SEMINAR ON THE GLOBAL WATER CRISIS 1:30 – 2:00 PM RECEPTION/MIXER 2:00 – 2:15 PM POETRY / SONG Mamade Kadreebux Sushmita Ghosh 2:15-4:00 PM SEMINAR INTRODUCTION Rosalie Say Welcome Founder’s Introduction: Mamade Kadreebux Welcome and Prefatory Remarks, Rash B. Ghosh, PhD, Founder, IIBHB SPECIAL WORDS FROM FRIENDS & WELL-WISHERS OF PROFESSOR CHARLES TOWNES 2:45 – 4:00 PM SESSION ONE The Convergence of Science and Spirituality David Presti, PhD, Professor, Molecular Cell Biology, UC Berkeley Water Budget Estimation and Water Management in the Mekong River Basin Jeanny Wang, President/Sr. Environmental Engineer, EcoWang Ltd. Sand from Newton’s Seashore: Introduction of Dr. Charles H. Townes John Paulin, PhD, Technical Writer and Editor, IIBHB Chief Guest Address: Vivekananda and a Vision for the South Asia, the US, and our Planet Charles H. Townes, PhD, 1964 Nobel Laureate in Physics, 1999 Rabindranath Tagore Award Recipient, and 2005 Templeton Prize Awardee Q & A 4:30 - 6:30 SESSION TWO INTRODUCTION OF KEYNOTE SPEAKER Derek Whitworth, PhD, President, IIBHB Keynote Address Steven Chu, 1997 Nobel Laureate in Physics and former U.S. Secretary of Energy Reducing the Impact of Toxics in Drinking Water Resources Rash B. Ghosh, PhD, Founder, IIBHB Special Presentation: How Advances in Science are Made. Douglas Osheroff, PhD, 1996 Nobel Laureate in Physics Q & A SUMMARY AND CONCLUDING REMARKS Sterling Bunnel, MD, IIBHB Former President and Advisor ACKNOWLEDGEMENT Master of Ceremonies Rosalie Say Professor Charles Hard Townes was born in 1915 and invented the microwave laser, or maser, in 1953 while at Columbia University. -

See the Scientific Petition
May 20, 2016 Implement the Endangered Species Act Using the Best Available Science To: Secretary Sally Jewell and Secretary Penny Prtizker We, the under-signed scientists, recommend the U.S. government place species conservation policy on firmer scientific footing by following the procedure described below for using the best available science. A recent survey finds that substantial numbers of scientists at the U.S. Fish and Wildlife Service (FWS) and the National Oceanic and Atmospheric Administration believe that political influence at their agency is too high.i Further, recent species listing and delisting decisions appear misaligned with scientific understanding.ii,iii,iv,v,vi For example, in its nationwide delisting decision for gray wolves in 2013, the FWS internal review failed the best science test when reviewed by an independent peer-review panel.vii Just last year, a FWS decision not to list the wolverine ran counter to the opinions of agency and external scientists.viii We ask that the Departments of the Interior and Commerce make determinations under the Endangered Species Actix only after they make public the independent recommendations from the scientific community, based on the best available science. The best available science comes from independent scientists with relevant expertise who are able to evaluate and synthesize the available science, and adhere to standards of peer-review and full conflict-of-interest disclosure. We ask that agency scientific recommendations be developed with external review by independent scientific experts. There are several mechanisms by which this can happen; however, of greatest importance is that an independent, external, and transparent science-based process is applied consistently to both listing and delisting decisions. -

Quiet Debut'' of the Double Helix: a Bibliometric and Methodological
Journal of the History of Biology Ó Springer 2009 DOI 10.1007/s10739-009-9183-2 Revisiting the ‘‘Quiet Debut’’ of the Double Helix: A Bibliometric and Methodological note on the ‘‘Impact’’ of Scientific Publications YVES GINGRAS De´partement d’histoire Universite´ du Que´bec a` Montre´al C.P. 8888, Suc. Centre-Ville Montreal, QC H3C-3P8 Canada E-mail: [email protected] Abstract. The object of this paper is two-fold: first, to show that contrary to what seem to have become a widely accepted view among historians of biology, the famous 1953 first Nature paper of Watson and Crick on the structure of DNA was widely cited – as compared to the average paper of the time – on a continuous basis from the very year of its publication and over the period 1953–1970 and that the citations came from a wide array of scientific journals. A systematic analysis of the bibliometric data thus shows that Watson’s and Crick’s paper did in fact have immediate and long term impact if we define ‘‘impact’’ in terms of comparative citations with other papers of the time. In this precise sense it did not fall into ‘‘relative oblivion’’ in the scientific community. The second aim of this paper is to show, using the case of the reception of the Watson–Crick and Jacob–Monod papers as concrete examples, how large scale bibliometric data can be used in a sophisticated manner to provide information about the dynamic of the scientific field as a whole instead of limiting the analysis to a few major actors and generalizing the result to the whole community without further ado. -

2005 Annual Report American Physical Society
1 2005 Annual Report American Physical Society APS 20052 APS OFFICERS 2006 APS OFFICERS PRESIDENT: PRESIDENT: Marvin L. Cohen John J. Hopfield University of California, Berkeley Princeton University PRESIDENT ELECT: PRESIDENT ELECT: John N. Bahcall Leo P. Kadanoff Institue for Advanced Study, Princeton University of Chicago VICE PRESIDENT: VICE PRESIDENT: John J. Hopfield Arthur Bienenstock Princeton University Stanford University PAST PRESIDENT: PAST PRESIDENT: Helen R. Quinn Marvin L. Cohen Stanford University, (SLAC) University of California, Berkeley EXECUTIVE OFFICER: EXECUTIVE OFFICER: Judy R. Franz Judy R. Franz University of Alabama, Huntsville University of Alabama, Huntsville TREASURER: TREASURER: Thomas McIlrath Thomas McIlrath University of Maryland (Emeritus) University of Maryland (Emeritus) EDITOR-IN-CHIEF: EDITOR-IN-CHIEF: Martin Blume Martin Blume Brookhaven National Laboratory (Emeritus) Brookhaven National Laboratory (Emeritus) PHOTO CREDITS: Cover (l-r): 1Diffraction patterns of a GaN quantum dot particle—UCLA; Spring-8/Riken, Japan; Stanford Synchrotron Radiation Lab, SLAC & UC Davis, Phys. Rev. Lett. 95 085503 (2005) 2TESLA 9-cell 1.3 GHz SRF cavities from ACCEL Corp. in Germany for ILC. (Courtesy Fermilab Visual Media Service 3G0 detector studying strange quarks in the proton—Jefferson Lab 4Sections of a resistive magnet (Florida-Bitter magnet) from NHMFL at Talahassee LETTER FROM THE PRESIDENT APS IN 2005 3 2005 was a very special year for the physics community and the American Physical Society. Declared the World Year of Physics by the United Nations, the year provided a unique opportunity for the international physics community to reach out to the general public while celebrating the centennial of Einstein’s “miraculous year.” The year started with an international Launching Conference in Paris, France that brought together more than 500 students from around the world to interact with leading physicists. -

Five Great Ideas of Biology
GREATGREAT IDEASIDEAS OFOF BIOLOGYBIOLOGY Paul Nurse KITP Public Lecture, Feb 24, 2010 THETHE CELLCELL The basic unit of life ROBERTROBERT HOOKEHOOKE’’SS MICROSCOPEMICROSCOPE Cork Image: Past Present STEMSTEM IMAGES:IMAGES: PASTPAST ANDAND PRESENTPRESENT Nehemiah Grew (1682) ANTONIANTONI VANVAN LEEUWENHOEKLEEUWENHOEK MICROORGANISMSMICROORGANISMS VANVAN LEEUWENHOEK?LEEUWENHOEK? THEODORTHEODOR SCHWANNSCHWANN “We have seen that all organisms are composed of essentially like parts, namely, of cells.” (1839) RUDOLFRUDOLF VIRCHOWVIRCHOW “Every animal appears as a sum of vital units, each of which bears in itself the complete characteristics of life.” (1858) CELLCELL Rockefeller Nobel Prize Winners in Cell Biology George E. Palade (1974) Christian de Duve (1974) Albert Claude (1974) Günter Blobel (1999) MAMMALIANMAMMALIAN EMBRYOEMBRYO SPERMSPERM ANDAND EGGEGG THETHE CELLCELL The basic unit of life Underpins all reproduction and development Stem cells THETHE GENEGENE Basis of heredity GREGORGREGOR MENDELMENDEL MENDELMENDEL’’SS GARDENGARDEN PEASPEAS PEASPEAS 1919TH CENTURYCENTURY CHROMOSOMESCHROMOSOMES EDOUARDEDOUARD VANVAN BENEDENBENEDEN’’SS NEMATODENEMATODE CHROMOSOMESCHROMOSOMES PNEUMOCOCCUSPNEUMOCOCCUS Avery, MacLeod and McCarty, Rockefeller University (1944) DNADNA MOLECULEMOLECULE CENTRALCENTRAL DOGMADOGMA THETHE GENEGENE Basis of heredity Genotype to phenotype Implications for what we are EVOLUTIONEVOLUTION BYBY NATURALNATURAL SELECTIONSELECTION Life evolves Mechanism of natural selection ERASMUSERASMUS ANDAND CHARLESCHARLES DARWINDARWIN -

Annual Report 2004
ANNUAL REPORT 2004 HELSINKI UNIVERSITY OF TECHNOLOGY Low Temperature Laboratory Brain Research Unit and Low Temperature Physics Research http://boojum.hut.fi - 2 - PREFACE...............................................................................................................5 SCIENTIFIC ADVISORY BOARD .......................................................................7 PERSONNEL .........................................................................................................7 SENIOR RESEARCHERS..................................................................................7 ADMINISTRATION AND TECHNICAL PERSONNEL...................................8 GRADUATE STUDENTS (SUPERVISOR).......................................................8 UNDERGRADUATE STUDENTS.....................................................................9 VISITORS FOR EU PROJECTS ......................................................................10 OTHER VISITORS ..........................................................................................11 GROUP VISITS................................................................................................13 OLLI V. LOUNASMAA MEMORIAL PRIZE 2004 ............................................15 INTERNATIONAL COLLABORATIONS ..........................................................16 CERN COLLABORATION (COMPASS) ........................................................16 COSLAB (COSMOLOGY IN THE LABORATORY)......................................16 ULTI III - ULTRA LOW TEMPERATURE INSTALLATION -

George Palade 1912-2008
George Palade, 1912-2008 Biography George Palade was born in November, 1912 in Jassy, Romania to an academic family. He graduated from the School of Medicine of the The Founding of Cell Biology University of Bucharest in 1940. His doctorial thesis, however, was on the microscopic anatomy of the cetacean delphinus Delphi. He The discipline of Cell Biology arose at Rockefeller University in the late practiced medicine in the second world war, and for a brief time af- 1940s and the 1950s, based on two complimentary techniques: cell frac- terwards before coming to the USA in 1946, where he met Albert tionation, pioneered by Albert Claude, George Palade, and Christian de Claude. Excited by the potential of the electron microscope, he Duve, and biological electron microscopy, pioneered by Keith Porter, joined the Rockefeller Institute for Medical Research, where he did Albert Claude, and George Palade. For the first time, it became possible his seminal work. He left Rockefeller in 1973 to chair the new De- to identify the components of the cell both structurally and biochemi- partment of Cell Biology at Yale, and then in 1990 he moved to the cally, and therefore begin understanding the functioning of cells on a University of California, San Diego as Dean for Scientific Affairs at molecular level. These individuals participated in establishing the Jour- the School of Medicine. He retired in 2001, at age 88. His first wife, nal of Cell Biology, (originally the Journal of Biochemical and Biophysi- Irina Malaxa, died in 1969, and in 1970 he married Marilyn Farquhar, cal Cytology), which later led, in 1960, to the organization of the Ameri- another prominent cell biologist, and his scientific collaborator. -
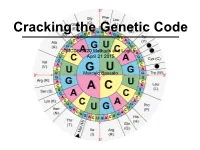
MCDB 5220 Methods and Logics April 21 2015 Marcelo Bassalo
Cracking the Genetic Code MCDB 5220 Methods and Logics April 21 2015 Marcelo Bassalo The DNA Saga… so far Important contributions for cracking the genetic code: • The “transforming principle” (1928) Frederick Griffith The DNA Saga… so far Important contributions for cracking the genetic code: • The “transforming principle” (1928) • The nature of the transforming principle: DNA (1944 - 1952) Oswald Avery Alfred Hershey Martha Chase The DNA Saga… so far Important contributions for cracking the genetic code: • The “transforming principle” (1928) • The nature of the transforming principle: DNA (1944 - 1952) • X-ray diffraction and the structure of proteins (1951) Linus Carl Pauling The DNA Saga… so far Important contributions for cracking the genetic code: • The “transforming principle” (1928) • The nature of the transforming principle: DNA (1944 - 1952) • X-ray diffraction and the structure of proteins (1951) • The structure of DNA (1953) James Watson and Francis Crick The DNA Saga… so far Important contributions for cracking the genetic code: • The “transforming principle” (1928) • The nature of the transforming principle: DNA (1944 - 1952) • X-ray diffraction and the structure of proteins (1951) • The structure of DNA (1953) How is DNA (4 nucleotides) the genetic material while proteins (20 amino acids) are the building blocks? ? DNA Protein ? The Coding Craze ? DNA Protein What was already known? • DNA resides inside the nucleus - DNA is not the carrier • Protein synthesis occur in the cytoplasm through ribosomes {• Only RNA is associated with ribosomes (no DNA) - rRNA is not the carrier { • Ribosomal RNA (rRNA) was a homogeneous population The “messenger RNA” hypothesis François Jacob Jacques Monod The Coding Craze ? DNA RNA Protein RNA Tie Club Table from Wikipedia The Coding Craze Who won the race Marshall Nirenberg J. -

Evidence for Design in Physics and Biology: from the Origin of the Universe to the Origin of Life
52 stephen c. meyer Pages 53–111 of Science and Evidence for Design in the Universe. The Proceedings of the Wethersfield Institute. Michael Behe, STEPHEN C. MEYER William A. Dembski, and Stephen C. Meyer (San Francisco: Ignatius Press, 2001. 2000 Homeland Foundation.) EVIDENCE FOR DESIGN IN PHYSICS AND BIOLOGY: FROM THE ORIGIN OF THE UNIVERSE TO THE ORIGIN OF LIFE 1. Introduction In the preceding essay, mathematician and probability theo- rist William Dembski notes that human beings often detect the prior activity of rational agents in the effects they leave behind.¹ Archaeologists assume, for example, that rational agents pro- duced the inscriptions on the Rosetta Stone; insurance fraud investigators detect certain ‘‘cheating patterns’’ that suggest intentional manipulation of circumstances rather than ‘‘natu- ral’’ disasters; and cryptographers distinguish between random signals and those that carry encoded messages. More importantly, Dembski’s work establishes the criteria by which we can recognize the effects of rational agents and distinguish them from the effects of natural causes. In brief, he shows that systems or sequences that are both ‘‘highly com- plex’’ (or very improbable) and ‘‘specified’’ are always produced by intelligent agents rather than by chance and/or physical- chemical laws. Complex sequences exhibit an irregular and improbable arrangement that defies expression by a simple formula or algorithm. A specification, on the other hand, is a match or correspondence between an event or object and an independently given pattern or set of functional requirements. As an illustration of the concepts of complexity and speci- fication, consider the following three sets of symbols: 53 54 stephen c. -

Works of Love
reader.ad section 9/21/05 12:38 PM Page 2 AMAZING LIGHT: Visions for Discovery AN INTERNATIONAL SYMPOSIUM IN HONOR OF THE 90TH BIRTHDAY YEAR OF CHARLES TOWNES October 6-8, 2005 — University of California, Berkeley Amazing Light Symposium and Gala Celebration c/o Metanexus Institute 3624 Market Street, Suite 301, Philadelphia, PA 19104 215.789.2200, [email protected] www.foundationalquestions.net/townes Saturday, October 8, 2005 We explore. What path to explore is important, as well as what we notice along the path. And there are always unturned stones along even well-trod paths. Discovery awaits those who spot and take the trouble to turn the stones. -- Charles H. Townes Table of Contents Table of Contents.............................................................................................................. 3 Welcome Letter................................................................................................................. 5 Conference Supporters and Organizers ............................................................................ 7 Sponsors.......................................................................................................................... 13 Program Agenda ............................................................................................................. 29 Amazing Light Young Scholars Competition................................................................. 37 Amazing Light Laser Challenge Website Competition.................................................. 41 Foundational