Trends in NMR Structural Elucidation of Polycyclic Cages, Namely: Adamantane, Pentacycloundecane and Trishomocubane
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
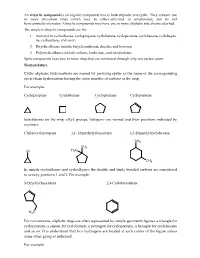
Nomenclature Cyclic Aliphatic Hydrocarbons Are Named By
An alicyclic compound is an organic compound that is both aliphatic and cyclic. They contain one or more all-carbon rings which may be either saturated or unsaturated, but do not have aromatic character. Alicyclic compounds may have one or more aliphatic side chains attached. The simplest alicyclic compounds are the 1. monocyclic cycloalkanes: cyclopropane, cyclobutane, cyclopentane, cyclohexane, cyclohepta ne, cyclooctane, and so on. 2. Bicyclic alkanes include bicycloundecane, decalin, and housane. 3. Polycyclic alkanes include cubane, basketane, and tetrahedrane. Spiro compounds have two or more rings that are connected through only one carbon atom. Nomenclature Cyclic aliphatic hydrocarbons are named by prefixing cyclo- to the name of the corresponding open-chain hydrocarbon having the same number of carbons as the ring. For example: Cyclopropane Cyclobutane Cyclopentane Cyclopentene Substituents on the ring- alkyl, groups, halogens- are named and their positions indicated by numbers. Chlorocyclopropane 1,1- Dimethylyclopentane 1,3-Dimethylcyclohexane CH3 CH3 Cl H3C CH3 In simple cycloalkenes and cycloalkynes the double and triply bonded carbons are considered to occupy positions 1 and 2. For example: 3-Ethylcyclopentene 1,3-Cyclohexadiene H3C For convenience, aliphatic rings are often represented by simple geometric figures: a triangle for cyclopropane, a square for cyclobutane, a pentagon for cyclopentane, a hexagon for cyclohexane and so on. It is understood that two hydrogens are located at each corner of the figure unless some other group is indicated. For example H3C cyclopentane 3-Ethylcyclopentene 1,3-Cyclopentadiene CH3 CH3 Cl CH Cyclohexane 3 1,3-Dimethylcyclohexane 2- Chloro-1-methylcyclohexane As usual alcohols are given the ending –ol, which takes priority over –ene and appears last in the name. -

The Smallest Quantum Vortex
“The 2D Symmetry of Nature” T.M. Lach April 11, 2005 The structures of nature and the strong nuclear force The Proton the smallest Quantum Vortex Over the years many new discoveries have taught us that nature works its miracles with very simple structures. Going back 100 years we see this simplicity in structures like the Benzene molecule, Polycyclic Aromatic Hydrocarbons (PAH), the Hydrogen atom, DNA, snowflakes and crystal lattices. (1) In recent years many new discoveries, like the discovery of Quarks, have reinforced this belief. Ninety years ago, Niels Bohr’s model of the structure of the hydrogen atom (March 6 th 1913) revolutionized science since it brought together a few simple principles that allowed us to understand the nature of why atoms have quantized energy levels. Thus began the new era of Quantum Theory and Quantum Mechanics. Many before Bohr had assumed that the electrons were circling the nucleus. (2) Nicholson earlier had assumed that the angular momentum of the electron was quantized, for 30 years others had tried to explain the Balmer series of the hydrogen spectrum, but Bohr tied it all together into a coherent picture of the hydrogen atom. Bohr had to assume that the circling electrons did not lose energy to radiation and thereby spiral into the nucleus, a big assumption at the time. Ten years later in 1923 DeBroglie proposed the wave nature of particles, which explained why the circling electrons could maintain stable quantized orbits and so began the quantum nature of the universe, with a little help from Plank, Einstein, Shrodinger, Sommerfeld and many others. -

Nomenclature – Naming of Organic Compounds
12/05/2020 Nomenclature –Naming of Organic Compounds 1 ‘Trivial’ Names… Aspirin Cubane Housane Basketane Olympicene 2 1 12/05/2020 IUPAC International Union of Pure and Applied Chemistry Pentacyclo[4.2.0.02,5.03,8.04,7]octane 3 Naming Organic Compounds Chains meth 1 C eth 2 C C prop 3 C C C but 4 CCCC pent 5 C C C C C hex 6 C C C C C C hept 7 C C C C C C C oct 8 C C C C C C C C non 9 C C C C C C C C C 4 2 12/05/2020 Chains – Numbering Positions of Substituents Cl C C C C C C C 2‐chloro… 1234567 Cl C C C C C C C 3‐chloro… 7 6 5 4 3 2 1 5 Single and Multiple Carbon‐Carbon Bonds in Chains C C C C C C Single carbon‐ Contains a Contains a an carbon bonds en carbon‐carbon yn carbon‐carbon only double bond triple bond C C C C C C C …‐1‐ene 1234567 C C C C C C C …‐2‐ene 1 234567 C C C C C C C …‐3‐ene 7 6 5 4 3 2 1 6 3 12/05/2020 Branches to Carbon Chains H H C CH3 methyl H Common H H branches H C C CH CH ethyl on chains: 3 2 H H H H H H C C C CH3 CH2CH2 propyl H H H 7 Position (3‐) Branch (methyl) Longest chain HHCH HHHH Length of longest chain (7 = hept) 3 Only single C‐C bonds (ane) H C C C C C C C H 1234567 3‐methylheptane HHHHHHH HHHHH H C C C C C H 3 4 5 6 HHHH 3‐methylhexane H C 2 H Longest chain H C1 H H 2‐methylhex‐2‐ene 8 4 12/05/2020 For each of the alkanes below, identify and highlight the longest unbroken chain. -

Cyclophane. Do MM Calculations of Each of These
10 52. Shown to the right are [2.2.2](1,3,5)-cyclophane and [2.2.2.2](1,2,3,5)-cyclophane. Do MM calculations of each of these as well as on all of the other two-carbon bridged structures: (1,2,3)-, (1,2,4)-, (1,2,3,4)-, (1,2,4,5)-, (1,2,3,4,5)-, and (1,2,3,4,5,6)-cyclophane; the latter is known as "superphane." Compare the computed angles and distances with those measured by X-ray crystallography (see the cited article). [Sekine, Y.; Boekelheide, V. J. Am. Chem. Soc. 1991, 103, 1777 and references cited therein; also Gleiter, R.; Kratz, D. Acc. Chem. Res. 1993, 26, 311.] 53. Shown to the right is [3.3](1,3)-cyclophane. There are at least five reasonable conformations for this compound (see the cited article). Use molecular mechanics to calculate the energy and geometry of each of these five conformations; assess which factors are responsible for the energy differences among the conformations. [Biali, S. E. J. Chem. Educ. 1990, 67, 1039.] 54. Use molecular mechanics to calculate the energies and geometries of a series of tetrasubstituted alkenes where R is H, Me, Et, iPr, or tBu. Compare your answers with those in Chart I and Table III of the first-cited reference. (Don't bother trying to reproduce the rotational barriers of Chart II because these are in doubt, as indicated by the second-cited reference.) [Clennan, E. L.; Chen, X.; Koola, J. J. J. Am. Chem. Soc. 1990, 112, 5193; Orfanopoulos, M.; Stratakis, M.; Elemes, Y.; Jensen, F. -

Chem 51A Chapter 4 2014
Lecture Notes Chem 51A S. King Chapter 4 Alkanes I. Alkanes – An Introduction Alkanes are aliphatic hydrocarbons having only C−H and C−C σ-bonds. They can be cyclic or acyclic. • Acyclic alkanes have the molecular formula CnH2n+2 (where n = an integer.) They are also called saturated hydrocarbons because they have the maximum number of hydrogen atoms per carbon. • Cyclic alkanes contain carbon atoms joined into a ring. They have molecular formula CnH2n. (Notice: 2 less hydrogens than saturated alkanes – Why? A. Unbranched Alkanes Alkanes with unbranched carbon chains are also known as normal alkanes or n- alkanes. The first four n-alkanes: H H H H H H H H H H H C H H C C H H C C C H H C C C C H H H H H H H H H H H • This is an example of a family of compounds known as homologous series (each member differs from the next by the addition of one methylene group (CH2). The first 12 n-alkanes are listed in the handout Essential Nomenclature. 71 Look at the various representations of propane: H H H H H H H C3H8 CH3CH2CH3 H C C C H C C H C H H H H H H H CH 3 H CH3 C H H H CH3 H H B. Branched Alkanes As the number of carbons of an alkane increase beyond three, the number of possible structures increases. An alkane with molecular formula C4H10, for example has two different ways to connect atoms together: CH3 CH2 CH2 CH3 CH3 CH CH3 CH3 These are examples of constitutional isomers: Compounds that have the same molecular formula but different connectivity. -

Chem 260 Handout 2013 Hydrocarbons
Hydrocarbons — Compounds that contain only Carbon and Hydrogen Types of hydrocarbons: Saturated: Alkanes only single, covalent C-C and C-H bonds, no rings Cycloalkanes same, but contain rings Unsaturated: Alkenes contain > 1 C=C double bond Alkynes contain > 1 C≡C triple bond Aromatic contain > 1 benzene ring General formula for alkanes is: CnH2n+2, n = 1, 2, 3 ... All carbons in alkanes are sp3 hybridized and tetrahedral (bond angles are 109.5°). The suffix "-ane" denotes an alkane. Be familiar with the variety of types of drawings of organic molecules. Know what atom is bonded to what atom. Dashes/wedges: recall that dashed bonds are going into the page, wedged bonds are coming out of the page, and lines are in the plane of the page. Line drawings: Each intersection or end of a line represents a carbon with the correct number of hydrogen atoms. Carbon always has four bonds in stable species. Hydrogens are often not drawn: be sure you know how many hydrogens are on each carbon: draw them in each time if it helps you. Be familiar with the following nomenclature: n = 1 methane n = 5 pentane n = 8 octane n = 2 ethane n = 6 hexane n = 9 nonane n = 3 propane n = 7 heptane n = 10 decane n = 4 butane Interlude: What do we have to know? Will this be on the exam? Anything could appear on an exam. But you should focus on what you really need to know. Know the drawings and nomenclature (don’t bother with index cards, most nomenclature comes just be doing homework). -

Cage Compounds with Main-Group Metals
Chem. Rev. 1990, 90. 3-16 3 Cage Compounds with Main-Group Metals MICHAEL VEITH linkorsitit des Searlandas, Anorganische chemic. -600 Saarbnkimn, Fedsra! Rep& of Gemvrny Recehd June 26. 1989 (Revlsed mnuscript Recaived October 9. 1989) Contents I. Introduction 11. Formation of Cages Inexporating Metals and Nonmetals A. The Principle of Lewis Acid-bse Interactions B. Orbital Participation at lhe Element 111. Cages with Metallic Elements A. Metal Derivatives of Organic Compounds E. Metal Amides 1. Silazane Cages Incorporating Lithium, Sodium, Magnesium. Calcium, and Aluminum 2. lminoalanes and Related Cages 8 3. Iminogermylenes. Iminostannylenes. 9 Iminoplumbylenes. and Related Cages Michael With was born in 1944 in Ooerlitz. Germany. He studied chemistry at tlw University 01 Munich, where he received hls DC 4. Cages with TI([). WIl). Sn(I1). 10 plodhemiker degree in 1969. Continuing wcfk with Prof. N. Nitrogen. and Oxygen as Main Wiberg, he received his doctoral degree in 1971. Ha men moved Components lo the University of Karlsruhe. where he started his postdoctoral C. Metal Alkoxides 11 work with Prof. H. Baernighausen. In 1977 he completed his 11 Habilitation in inorganic chemistry. Until the end of 1978 he was 1. Cages Formed by Metal Alkoxides and Privat-Dozent at the University of Karlsruhe. In 1979 he moved Related Compounds lo the Technische Universitat Braunschweig as a professor of 2. Cages Incorporating WII). Sn(Il), or 13 inorganic chemistry. In 1984 he received offers for a full pro- Pb(l1). Metal AlkoxMes, and Main-Group fessorship at the University of Oldenburg. the University of He!- Metals delkg. and We UnivmiIy of Saarland (Saarbrijcken). -

Xerox University Microfilms
INFORMATION TO USERS This material was produced from a microfilm copy of the original document. While the most advanced technological means to photograph and reproduce this document have been used, the quality is heavily dependent upon the quality of the original submitted. The following explanation of techniques is provided to help you understand markings or patterns which may appear on this reproduction. 1. The sign or "target" for pages apparently lacking from the document photographed is "Missing Paga(s)". If it was possible to obtain the missing page(s) or section, they are spliced into the film along with adjacent pages. This may have necessitated cutting thru an image and duplicating adjacent pages to insure you complete continuity. 2. Whan an image on the film is obliterated with a large round black mark, it is an indication that the photographer suspected that the copy may have moved during exposure and thus cause a blurred image. You will find a good image of the paga in the adjacent frame. 3. When a map, drawing or chart, etc., was part of the material being photographed the photographer followed a definite method in "sectioning" the material. It is customary to begin photoing at the upper left hand corner of a large sheet and to continue photoing from left to right in equal sections with a small overlap. If necessary, sectioning is continued again — beginning below the first row and continuing on until complete.' 4. The majority of users indicate that the textual content is of greatest value, however, a somewhat higher quality reproduction could be made from "photographs" if essential to the understanding of the dissertation. -

Organic Chemistry I
Organic Chemistry I Mohammad Jafarzadeh Faculty of Chemistry, Razi University Organic Chemistry, Structure and Function (7th edition) By P. Vollhardt and N. Schore, Elsevier, 2014 1 2-4 Functional Groups: Centers of Reactivity CHAPTER 2 69 by an acid dissociation constant Ka. Removal of a proton from an acid generates its conju- gate base; attachment of a proton to a base forms its conjugate acid. Lewis bases donate an electron pair to form a covalent bond with Lewis acids, a process depicted by a curved arrow pointing from the lone pair of the base toward the acid. Electrophiles and nucleo- philes are species in organic chemistry that interact very much like acids and bases. The carbon–halogen bond in the haloalkane is its functional group. It contains an electrophilic carbon atom, which reacts with nucleophiles in a process called nucleophilic substitution. 2-4 FUNCTIONAL GROUPS: CENTERS OF REACTIVITY Many organic molecules consist predominantly of a backbone of carbons linked by single bonds, Manywith only organichydrogen moleculesatoms consistattached predominantly. They ofmay a backbonealso contain of carbonsdoubly linkedor triplyby singlebonded carbons,bonds,as well withas onlyother hydrogenelements atoms. attached. However, they may also contain doubly or R Function triply bonded carbons, as well as other elements. These atoms or groups of atoms tend to These atomsbe sitesor of groupscomparativelyof atoms high tendchemicalto be reactivitysites of andcomparatively are referred to highas functionalchemical groupsreactivity and areorreferred functionalities.to as functional Such groupsgroups have characteristicor functionalities properties,. and they control the reactiv- Carbon frame Functional ity of the molecule as a whole. provides group imparts Such groups have characteristic properties, and they control the reactivity of the molecule structure reactivity as a wholeHydrocarbons. -

Heteroatom Effects on 2, 8-Annulated Semibullvalene Equilibria
INFORMATION TO USERS This material was produced from a microfilm copy of the original document. While the most advanced technological means to photograph and reproduce this document have been used, the quality is heavily dependent upon the quality of the original submitted. The following explanation of techniques is provided to help you understand markings or patterns which may appear on this reproduction. 1. The sign or "target" for pages apparently lacking from the document photographed is "Missing Page(s)". If it was possible to obtain the missing page(s) or section, they are spliced into the film along with adjacent pages. This may have necessitated cutting thru an image and duplicating adjacent pages to insure you complete continuity. 2. When an image on the film is obliterated with a large round black mark, it is an indication that the photographer suspected that the copy may have moved during exposure and thus cause a blurred image. You will find a good image of the page in the adjacent frame. 3. When a map, drawing or chart, etc., was part of the material being photographed the photographer followed a definite method in "sectioning" the material. It is customary to begin photoing at the upper left hand corner of a large sheet and to continue photoing from left to right in equal sections with a small overlap. If necessary, sectioning is continued again - beginning below the first row and continuing on until complete. 4. The majority of users indicate that the textual content is of greatest value, however, a somewhat higher quality reproduction could be made from "photographs" if essential to the understanding of the dissertation. -
![11 Nomenclature [Tryb Zgodności]](https://docslib.b-cdn.net/cover/0869/11-nomenclature-tryb-zgodno%C5%9Bci-9710869.webp)
11 Nomenclature [Tryb Zgodności]
2015-01-14 Webinar ACS: http://www.acs.org/content/acs/en/events/upcoming-acs-webinars/lab-mistakes.html Strona dr. Alice Frontier: Not Voodoo: Demystifying Synthetic Organic Chemistry http://chem.chem.rochester.edu/~nvd/?page=home Rookie mistakes: http://chem.chem.rochester.edu/~nvd/pages/rookie-mistakes.php?page=main Chemical compounds and their names 21.10.2013 1 2015-01-14 Elements 1. iron 11. chlorine 2. oxygen 12. bromine 3. sulphur 13. barium 4. nitrogen 14. calcium 5. tin 15. phosphorus 6. tungsten 16. arsenic 7. copper 17. potassium 8. hydrogen 18. carbon 9. lithium 19. boron 10. mercury 20. aluminium Inorganic compounds • oxides • carbon monooxide • acids • sulphuric acid/sulfuric acid • bases • sodium hydroxide • salts • potassium chloride 2 2015-01-14 Main Entry: al·kane Pronunciation: 'al-"kAn : any of numerous saturated hydrocarbons; specifically : any of a series of open-chain hydrocarbons C nH2n+2 (as methane and butane) -- called also paraffin Main Entry: al·kene Pronunciation: 'al-"kEn : any of numerous unsaturated hydrocarbons having one double bond; specifically : any of a series of open-chain hydrocarbons C nH2n (as ethylene) Main Entry: al·kyne Pronunciation: 'al-"kIn : any of a series of open-chain hydrocarbons C nH2n-2 (as acetylene) having one triple bond Origins of Common Names of Organic Compounds Hydrocarbon Root Names Methane from methyl, Greek methy = wine + hyle = wood ( i.e. , wood alcohol) Ethane from ethyl, German Athyl , from Ather = ether, Latin aether = to light up Propane from propionic (see below) Butane from butyric (see below) The rest, of course, have numeric roots 3 2015-01-14 The IUPAC system requires first that we have names for simple unbranched chains, as noted above, and second that we have names for simple alkyl groups that may be attached to the chains. -

Organic Chemistry
SIXTH EDITION Organic Chemistry Robert Thornton Morrison Robert Neilson Boyd New York University Prentice Hall, Englewood Clzffs,New Jersey 0 7632 Contents Preface xxiii Acknowledgments xxvii PART ONE The Fundamentals l Structure and Properties Organic chemistry 1 The structural theory 3 The chemical bond before 1926 4 Quantum mechanics 5 Atomic orbitals 6 Electronic configuration. Pauli exclusion principle 8 Molecular orbitals 9 The covalent bond 9 Hybrid orbitals: sp l l Hybrid orbitals: sp 13 Hybrid orbitals: sp3 15 Unshared pairs of electrons 17 Intramolecular forces 20 Bond dissociation energy. Homolysis and heterolysis 21 Polarity of bonds 23 Polarity of molecules 23 Structure and physical properties 26 Melting point 2 7 Intermolecular forces 28 Boiling point 30 Solubility 31 vi CONTENTS 1.22 Acids and bases 33 1.23 Isomerism 36 2 Methane Energy of Activation. Transition State Hydrocarbons 39 Structure of methane 40 Physical properties 41 Source 41 Reactions 42 Oxidation. Heat of combustion 42 Chlorination: a substitution reaction 43 Control of chlorination 44 Reaction with other halogens: halogenation 44 Relative reactivity 45 Reaction mechanisms 45 Mechanism of chlorination. Free radicals 46 Chain reactions 48 Inhibitors 49 Heat of reaction 50 Energy of activation 51 Progress of reaction: energy changes 52 Rate of reaction 55 Relative rates of reaction 58 Relative reactivities of halogens toward methane 59 An alternative mechanism for halogenation 61 Structure of the methyl radical. sp2Hybridization 64 Transition state 65 Reactiyity and development of the transition state 67 Chlorofluorocarbons and the ozone shield 69 Molecular formula: its hndamental importance 72 Qualitative elemental analysis 72 . Quantitative elemental analysis: carbon, hydrogen, and halogen 73 Empirical formula 74 Molecular weight.