Xerox University Microfilms , Ann Arbor, Michigan 48106
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
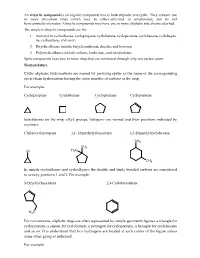
Nomenclature Cyclic Aliphatic Hydrocarbons Are Named By
An alicyclic compound is an organic compound that is both aliphatic and cyclic. They contain one or more all-carbon rings which may be either saturated or unsaturated, but do not have aromatic character. Alicyclic compounds may have one or more aliphatic side chains attached. The simplest alicyclic compounds are the 1. monocyclic cycloalkanes: cyclopropane, cyclobutane, cyclopentane, cyclohexane, cyclohepta ne, cyclooctane, and so on. 2. Bicyclic alkanes include bicycloundecane, decalin, and housane. 3. Polycyclic alkanes include cubane, basketane, and tetrahedrane. Spiro compounds have two or more rings that are connected through only one carbon atom. Nomenclature Cyclic aliphatic hydrocarbons are named by prefixing cyclo- to the name of the corresponding open-chain hydrocarbon having the same number of carbons as the ring. For example: Cyclopropane Cyclobutane Cyclopentane Cyclopentene Substituents on the ring- alkyl, groups, halogens- are named and their positions indicated by numbers. Chlorocyclopropane 1,1- Dimethylyclopentane 1,3-Dimethylcyclohexane CH3 CH3 Cl H3C CH3 In simple cycloalkenes and cycloalkynes the double and triply bonded carbons are considered to occupy positions 1 and 2. For example: 3-Ethylcyclopentene 1,3-Cyclohexadiene H3C For convenience, aliphatic rings are often represented by simple geometric figures: a triangle for cyclopropane, a square for cyclobutane, a pentagon for cyclopentane, a hexagon for cyclohexane and so on. It is understood that two hydrogens are located at each corner of the figure unless some other group is indicated. For example H3C cyclopentane 3-Ethylcyclopentene 1,3-Cyclopentadiene CH3 CH3 Cl CH Cyclohexane 3 1,3-Dimethylcyclohexane 2- Chloro-1-methylcyclohexane As usual alcohols are given the ending –ol, which takes priority over –ene and appears last in the name. -

The Smallest Quantum Vortex
“The 2D Symmetry of Nature” T.M. Lach April 11, 2005 The structures of nature and the strong nuclear force The Proton the smallest Quantum Vortex Over the years many new discoveries have taught us that nature works its miracles with very simple structures. Going back 100 years we see this simplicity in structures like the Benzene molecule, Polycyclic Aromatic Hydrocarbons (PAH), the Hydrogen atom, DNA, snowflakes and crystal lattices. (1) In recent years many new discoveries, like the discovery of Quarks, have reinforced this belief. Ninety years ago, Niels Bohr’s model of the structure of the hydrogen atom (March 6 th 1913) revolutionized science since it brought together a few simple principles that allowed us to understand the nature of why atoms have quantized energy levels. Thus began the new era of Quantum Theory and Quantum Mechanics. Many before Bohr had assumed that the electrons were circling the nucleus. (2) Nicholson earlier had assumed that the angular momentum of the electron was quantized, for 30 years others had tried to explain the Balmer series of the hydrogen spectrum, but Bohr tied it all together into a coherent picture of the hydrogen atom. Bohr had to assume that the circling electrons did not lose energy to radiation and thereby spiral into the nucleus, a big assumption at the time. Ten years later in 1923 DeBroglie proposed the wave nature of particles, which explained why the circling electrons could maintain stable quantized orbits and so began the quantum nature of the universe, with a little help from Plank, Einstein, Shrodinger, Sommerfeld and many others. -

Xarox University Microfilms
INFORMATION TO USERS Thil material was produced from a microfilm copy of the original documant. While the moat advanced technological meant to photograph and reproduce this documant have been used, the quality is heavily dependant upon the quality of the original submitted. The following explanation o f techniques is provided to help you understand markings or patterns which m ay appear on this reproduction. 1. The sign or "target" for pages apparently lacking from the document photographed is "Misting Page(s)". If it was possible to obtain the misting page(s) or section, th a y era spliced into the film along with adjacent pages. This may have necessitated cutting thru an image and duplicating adjacent pagae to insure you complete continuity. 2. Whan an image on th e film is obliterated with a large round black mark, it is an indication that the photographer suspected that the copy may have moved during exposure and thus causa a blurred image. You will find a good image of the pnga in the adjacent frame. 3. Whan a map, drawing or chart, etc., was part of the material being photographed the photographer followed a definite method in "sectioning" the material. It ie customary to begin photoing at the upper left hand comer of a large sheet and to continue photoing from left to right in equal sections with a small overlap. If necessary, sectioning is continued again — beginning below the first row end continuing on until complete. 4. The majority of users indicate that the textual content is of greatest value, however, a somewhat higher quality reproduction could be made from "photographs" if essential to the understanding of the dissertation. -

37Th Rocky Mountain Conference on Analytical Chemistry
Rocky Mountain Conference on Magnetic Resonance Volume 37 37th Rocky Mountain Conference on Article 1 Analytical Chemistry July 1995 37th Rocky Mountain Conference on Analytical Chemistry Follow this and additional works at: https://digitalcommons.du.edu/rockychem Part of the Chemistry Commons, Materials Science and Engineering Commons, and the Physics Commons Recommended Citation (1995) "37th Rocky Mountain Conference on Analytical Chemistry," Rocky Mountain Conference on Magnetic Resonance: Vol. 37 , Article 1. Available at: https://digitalcommons.du.edu/rockychem/vol37/iss1/1 This work is licensed under a Creative Commons Attribution 4.0 License. This Article is brought to you for free and open access by Digital Commons @ DU. It has been accepted for inclusion in Rocky Mountain Conference on Magnetic Resonance by an authorized editor of Digital Commons @ DU. For more information, please contact [email protected],dig- [email protected]. et al.: 37th RMCAC Final Program and Abstracts 37TH ROCKY MOUNTAIN CONFERENCE ON ANALYTICAL CHEMISTRY FINAL PROGRAM AND ABSTRACTS JULY 23-27, 1995 HYATT REGENCY DENVER 1750 WELTON STREET DENVER, COLORADO SPONSORED BY: ROCKY MOUNTAIN SECTION SOCIETY FOR APPLIED SPECTROSCOPY & COLORADO SECTION AMERICAN CHEMICAL SOCIETY Published by Digital Commons @ DU, 1995 1 Rocky Mountain Conference on Magnetic Resonance, Vol. 37 [1995], Art. 1 TABLE OF CONTENTS Conference Organizers 2 Symposia Organizers 3 Registration and Event Information 5 Exhibitor List 8 Hotel and Visitor Information 9 Employment Clearing House and Professional Memberships 9 Short Courses 10 Vendor Workshops 12 Symposia Schedule: Atomic Spectroscopy 17 Chromatography 18 Compost (Biogeochemistry of) 19 Electrochemistry 20 Environmental Chemistry 22 EPR 25 FTIR/NIR/RAMAN Spectroscopy 37 General Posters 37 ICP-MS 39 Laboratory Safety 41 Luminescence 42 Mass Spectrometry 44 NMR 47 Pharmaceutical Analysis 59 Quality Assurance 60 Downtown Denver Street Map Inside back cover Abstracts start after page 61. -

Nomenclature – Naming of Organic Compounds
12/05/2020 Nomenclature –Naming of Organic Compounds 1 ‘Trivial’ Names… Aspirin Cubane Housane Basketane Olympicene 2 1 12/05/2020 IUPAC International Union of Pure and Applied Chemistry Pentacyclo[4.2.0.02,5.03,8.04,7]octane 3 Naming Organic Compounds Chains meth 1 C eth 2 C C prop 3 C C C but 4 CCCC pent 5 C C C C C hex 6 C C C C C C hept 7 C C C C C C C oct 8 C C C C C C C C non 9 C C C C C C C C C 4 2 12/05/2020 Chains – Numbering Positions of Substituents Cl C C C C C C C 2‐chloro… 1234567 Cl C C C C C C C 3‐chloro… 7 6 5 4 3 2 1 5 Single and Multiple Carbon‐Carbon Bonds in Chains C C C C C C Single carbon‐ Contains a Contains a an carbon bonds en carbon‐carbon yn carbon‐carbon only double bond triple bond C C C C C C C …‐1‐ene 1234567 C C C C C C C …‐2‐ene 1 234567 C C C C C C C …‐3‐ene 7 6 5 4 3 2 1 6 3 12/05/2020 Branches to Carbon Chains H H C CH3 methyl H Common H H branches H C C CH CH ethyl on chains: 3 2 H H H H H H C C C CH3 CH2CH2 propyl H H H 7 Position (3‐) Branch (methyl) Longest chain HHCH HHHH Length of longest chain (7 = hept) 3 Only single C‐C bonds (ane) H C C C C C C C H 1234567 3‐methylheptane HHHHHHH HHHHH H C C C C C H 3 4 5 6 HHHH 3‐methylhexane H C 2 H Longest chain H C1 H H 2‐methylhex‐2‐ene 8 4 12/05/2020 For each of the alkanes below, identify and highlight the longest unbroken chain. -

Cyclophane. Do MM Calculations of Each of These
10 52. Shown to the right are [2.2.2](1,3,5)-cyclophane and [2.2.2.2](1,2,3,5)-cyclophane. Do MM calculations of each of these as well as on all of the other two-carbon bridged structures: (1,2,3)-, (1,2,4)-, (1,2,3,4)-, (1,2,4,5)-, (1,2,3,4,5)-, and (1,2,3,4,5,6)-cyclophane; the latter is known as "superphane." Compare the computed angles and distances with those measured by X-ray crystallography (see the cited article). [Sekine, Y.; Boekelheide, V. J. Am. Chem. Soc. 1991, 103, 1777 and references cited therein; also Gleiter, R.; Kratz, D. Acc. Chem. Res. 1993, 26, 311.] 53. Shown to the right is [3.3](1,3)-cyclophane. There are at least five reasonable conformations for this compound (see the cited article). Use molecular mechanics to calculate the energy and geometry of each of these five conformations; assess which factors are responsible for the energy differences among the conformations. [Biali, S. E. J. Chem. Educ. 1990, 67, 1039.] 54. Use molecular mechanics to calculate the energies and geometries of a series of tetrasubstituted alkenes where R is H, Me, Et, iPr, or tBu. Compare your answers with those in Chart I and Table III of the first-cited reference. (Don't bother trying to reproduce the rotational barriers of Chart II because these are in doubt, as indicated by the second-cited reference.) [Clennan, E. L.; Chen, X.; Koola, J. J. J. Am. Chem. Soc. 1990, 112, 5193; Orfanopoulos, M.; Stratakis, M.; Elemes, Y.; Jensen, F. -

Synthesis and Reactions of 4,5-Homotropone and 4,5-Dimethylenetropone Richard Anthony Fugiel Iowa State University
Iowa State University Capstones, Theses and Retrospective Theses and Dissertations Dissertations 1974 Synthesis and reactions of 4,5-homotropone and 4,5-dimethylenetropone Richard Anthony Fugiel Iowa State University Follow this and additional works at: https://lib.dr.iastate.edu/rtd Part of the Organic Chemistry Commons Recommended Citation Fugiel, Richard Anthony, "Synthesis and reactions of 4,5-homotropone and 4,5-dimethylenetropone " (1974). Retrospective Theses and Dissertations. 5986. https://lib.dr.iastate.edu/rtd/5986 This Dissertation is brought to you for free and open access by the Iowa State University Capstones, Theses and Dissertations at Iowa State University Digital Repository. It has been accepted for inclusion in Retrospective Theses and Dissertations by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. INFORIVIATIOIM TO USERS This material was produced from a microfilm copy of the original document. While the most advanced technological means to photograph and reproduce this document have been used, the quality is heavily dependent upon the quality of the original submitted. The following explanation of techniques is provided to help you understand markings or patterns which may appear on this reproduction. 1.The sign or "target" for pages apparently lacking from the document photographed is "Missing Page(s)". If it was possible to obtain the missing page(s) or section, they are spliced into the film along with adjacent pages. This may have necessitated cutting thru an image and duplicating adjacent pages to insure you complete continuity. 2. When an image on the film is obliterated with a large round black mark, it is an indication that the photographer suspected that the copy may have moved during exposure and thus cause a blurred image. -

Information to Users
INFORMATION TO USERS This manuscript has been reproduced from the microfilm master. UMI films the text directly from the original or copy submitted. Thus, some thesis and dissertation copies are in typewriter face, while others may be from any type of computer printer. The quality of this reproduction is dependent upon the quality of the copy submitted. Broken or indistinct print, colored or poor quality illustrations and photographs, print bleedthrough, substandard margins, and improper alignment can adversely affect reproduction. In the unlikely event that the author did not send UMI a complete manuscript and there are missing pages, these will be noted. Also, if unauthorized copyright material had to be removed, a note will indicate the deletion. Oversize materials (e.g., maps, drawings, charts) are reproduced by sectioning the original, beginning at the upper left-hand corner and continuing from left to right in equal sections with small overlaps. Each original is also photographed in one exposure and is included in reduced form at the back of the book. Photographs included in the original manuscript have been reproduced xerographically in this copy. Higher quality 6" x 9" black and white photographic prints are available for any photographs or illustrations appearing in this copy for an additional charge. Contact UMI directly to order. University Microfilms international A Bell & Howell Information Company 300 North ZeebRoad Ann Arbor Ml 48106-1346 USA 313/761-4700 800 521-0600 Order Number 9427071 The chemistry of polycyclic and spirocyclic compounds Branan, Bruce Monroe, Ph.D. The Ohio State University, 1994 UMI 300 N.ZeebRd. Ann Arbor, MI 48106 THE CHEMISTRY OF POLYCYCLIC AND SPIROCYCLIC COMPOUNDS DISSERTATION Presented in Partial Fulfillment of the Requirements for the Degree Doctor of Philosophy in the Graduate School of The Ohio State University by Bruce Monroe Branan ***** The Ohio State University 1994 Dissertation Committee: Approved by Professor Leo A. -

The Synthesis of Carbacyclic Silanes Via Organosilicon Reactive Intermediates Michael J
Iowa State University Capstones, Theses and Retrospective Theses and Dissertations Dissertations 1984 The synthesis of carbacyclic silanes via organosilicon reactive intermediates Michael J. Vuper Iowa State University Follow this and additional works at: https://lib.dr.iastate.edu/rtd Part of the Organic Chemistry Commons Recommended Citation Vuper, Michael J., "The synthesis of carbacyclic silanes via organosilicon reactive intermediates " (1984). Retrospective Theses and Dissertations. 7737. https://lib.dr.iastate.edu/rtd/7737 This Dissertation is brought to you for free and open access by the Iowa State University Capstones, Theses and Dissertations at Iowa State University Digital Repository. It has been accepted for inclusion in Retrospective Theses and Dissertations by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. INFORMATION TO USERS Tliis reproduction was made from a copy of a document sent to us for microfilming. While the most advanced technology has been used to photograph and reproduce this document, the quality of the reproduction is heavily dependent upon the quality of the material submitted. The following explanation of techniques is provided to help clarify markings or notations which may appear on this reproduction. 1. The sign or "target" for pages apparently lacking from the document photographed is "Missing Page(s)". If it was possible to obtain the missing page(s) or section, they are spliced into the film along with adjacent pages. This may have necessitated cutting through an image and duplicating adjacent pages to assure complete continuity. 2. When an image on the film is obliterated with a round black mark, it is an indication of either blurred copy because of movement during exposure, duplicate copy, or copyrighted materials that should not have been filmed. -

William Von Eggers Doering's Remarkable Career - Personal Observations from the 1950S and '60S
William von Eggers Doering's Remarkable Career - Personal Observations from the 1950s and '60s "... the questions themselves" Rainer Maria Rilke RONALD M. MAGID AND MAITLAND JONES, JR. Department of Chemistry, The University of Tennessee; Department of Chemistry, New York University Received Dedicated to Professor Bill Doering on the occasion of his 90th birthday ______________________________________________________________ ABSTRACT This Account consists of reminiscences about the Doering research group at Yale University, nearly a half-century ago. We recount vignettes and events that reflect the state of research in physical organic chemistry at that early time, the passion that drove Doering, and the lessons that he taught to his coworkers. We allude to specific research results that are described in considerable detail in the preceding article. _________________________________________________________________ In the accompanying article1 we describe some of the truly remarkable research accomplishments by Bill Doering and his coworkers over a 60-year-span. An editor, who may yet regret it, thought that it would be interesting to include some reminiscences by two of Bill's former graduate students. It was suggested that they might focus on the human side of the equation: what made Doering tick, how did the research group function, what was the nature of physical organic chemistry research and equipment in the 1950s-60s, etc. RMM and MJJr are the co-authors of this Account. Both were Yale undergraduates in Bill's introductory organic course in Spring-Fall, 1957, and both did their graduate work under Bill's supervision. MJJr's knowledge of Doering both precedes and supercedes the 1957-1963 period. Even before college, he had spent some summers at Hickrill Chemical Research Laboratory (about which much more, soon). -

Organic Chemistry Masterclasses
Organic Chemistry Masterclasses By S. Ranganathan All rights reserved. No parts of this publication may be reproduced, stored in a retrieval system, or transmitted, in any form or by any means, electronic, mechanical, photocopy- ing, recording, or otherwise, without prior permission of the publisher. © Indian Academy of Sciences Published by Indian Academy of Sciences 2 Foreword The Masterclass series of eBooks published by the Indian Academy of Sciences, Bengaluru brings together pedagogical articles on single broad topics reproduced from Resonance, Journal of Science Education that is published monthly by the Academy since January 1996. Primarily directed at students and teachers at the undergraduate level, the journal has brought out a wide spectrum of articles in a range of scientific disciplines. Articles in the journal are written in a style that makes them appealing to readers from diverse backgrounds, and in addition, they provide a useful source of instruction that is not always available in textbooks. It is fitting that the first book in this series,Organic Chemistry Masterclasses, is by a legendary teacher, Professor Subramania Ranganathan, highly acclaimed and distinguished Organic Chemist. Ranga, as he is fondly known to generations of students at the IIT Kanpur where he taught for over three decades, has been a regular contributor to Resonance and his articles enjoy wide popularity among the journal’s readership. Indeed his first contribution toResonance was in the very first issue of the journal. We are also delighted that this collection of over fifteen articles, reformatted as an eBook will mark his 80th birthday. Those of us who were fortunate to have learned some of these topics as he was devising ways of teaching them will be very pleased that some of Ranga’s masterly instructions can reach a much wider audience through this means. -

Chem 51A Chapter 4 2014
Lecture Notes Chem 51A S. King Chapter 4 Alkanes I. Alkanes – An Introduction Alkanes are aliphatic hydrocarbons having only C−H and C−C σ-bonds. They can be cyclic or acyclic. • Acyclic alkanes have the molecular formula CnH2n+2 (where n = an integer.) They are also called saturated hydrocarbons because they have the maximum number of hydrogen atoms per carbon. • Cyclic alkanes contain carbon atoms joined into a ring. They have molecular formula CnH2n. (Notice: 2 less hydrogens than saturated alkanes – Why? A. Unbranched Alkanes Alkanes with unbranched carbon chains are also known as normal alkanes or n- alkanes. The first four n-alkanes: H H H H H H H H H H H C H H C C H H C C C H H C C C C H H H H H H H H H H H • This is an example of a family of compounds known as homologous series (each member differs from the next by the addition of one methylene group (CH2). The first 12 n-alkanes are listed in the handout Essential Nomenclature. 71 Look at the various representations of propane: H H H H H H H C3H8 CH3CH2CH3 H C C C H C C H C H H H H H H H CH 3 H CH3 C H H H CH3 H H B. Branched Alkanes As the number of carbons of an alkane increase beyond three, the number of possible structures increases. An alkane with molecular formula C4H10, for example has two different ways to connect atoms together: CH3 CH2 CH2 CH3 CH3 CH CH3 CH3 These are examples of constitutional isomers: Compounds that have the same molecular formula but different connectivity.