Stereochemistry of Organic Molecules SECOND EDITION Press
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Enantiotopicity, Diastereotopicity
Stereochemistry and stereocontrolled synthesis (OC 8) A lecture from Prof. Paul Knochel, Ludwig-Maximilians-Universität München WS 2015-16 1 Wichtig! • Prüfung Stereochemistry 02. Februar 2016 8:00 – 10:00 Willstätter-HS • Nachholklausur Stereochemistry 5. April 2016 9:00 – 11:00 Willstätter-HS 2 Problem set part I 3 Problem set part II 4 Problem set part III 5 Recommended Literature • E. Juaristi, Stereochemistry and Conformational Analysis, Wiley, 1991. • E. Eliel, Stereochemistry of Organic Compounds, Wiley, 1994. • A. Koskinen, Asymmetric Synthesis of Natural Products, Wiley, 1993. • R. Noyori, Asymmetric Catalysis, Wiley, 1994. • F. A. Carey, R. J. Sundberg, Advanced Organic Chemistry, 5th Edition, Springer, 2007. • A. N. Collins, G. N. Sheldrake, J. Crosby, Chirality in Industrie, Vol. I and II, Wiley, 1995 and 1997. • G.Q. Lin, Y.-M. Li, A.S.C. Chan, Asymmetric Synthesis, 2001, ISBN 0-471-40027-0. • P. Deslongchamps, Stereoelectronic Effects in Organic Chemistry, Pergamon, 1983. • M. Nogradi, Stereoselective Synthesis, VCH, 1995. • E. Winterfeldt, Stereoselective Synthese, Vieweg, 1988. • R. Mahrwald (Ed.), Modern Aldol Reactions, Vol. I and II, Wiley, 2004. • C. Wolf, Dynamic Stereochemistry of Chiral Compounds, RSC Publishing, 2008. • A. Berkessel, H. Gröger, Asymmetric Organocatalysis, Wiley-VCH, 2005. • J. Christoffers, A. Baro (Eds.), Quaternary Stereocenters, Wiley-VCH, 2005. • Catalytic Asymmetric Synthesis, I. Oshima (Ed.), Wiley, 2010. 6 Recent advances of asymmetric catalysis 7 Asymmetric Hydrogenation of Heterocyclic Compounds 8 R. Kuwano, N. Kameyama, R. Ikeda, J. Am. Chem. Soc. 2011, 133, 7312-7315. Camphor-Derived Organocatalytic Synthesis of Chromanones 9 Z.-Q. Rong, Y. Li, G.-Q. Yang, S.-L. You, Synlett 2011, 1033-1037. -

Organic Chemistry –II
Subject Chemistry Paper No and Title Paper 5: Organic Chemistry –II Module No and Title Module 5: Methods of determining mechanisms and Isotope effects Module Tag CHE_P5_M5_e-Text CHEMISTRY PAPER No. 5: Organic Chemistry -II MODULE No. 5: Methods of determining mechanisms and Isotope effects TABLE OF CONTENTS 1. Learning Outcomes 2. Introduction 3. Methods of determining mechanism 3.1 Determination of the products formed 3.2 Study of Intermediate formed 3.3 Study of catalyst 3.4 Stereochemical Evidence 3.5 Kinetic evidence 3.6 Isotope Labelling 4. Isotopic Effects 5. Summary CHEMISTRY PAPER No. 5: Organic Chemistry -II MODULE No. 5: Methods of determining mechanisms and Isotope effects 1. Learning Outcomes After studying this module, you shall be able to • Know what do we mean by mechanism of a reaction • Learn the ways to determine mechanism of a reaction • Identify the reactants, products and intermediates involved in a reaction • Evaluate the steps involved in a reaction 2. Introduction In scientific experiments and chemical reactions, all we can do is try to account for the observations by proposing theories and mechanisms. Reaction mechanisms have been an integral part of the teaching of organic chemistry and in the planning of routes for organic syntheses for about 50 years. The first sentence of Hammett’s influential book, Physical Organic Chemistry, states, “A major part of the job of the chemist is the prediction and control of the course of chemical reactions” In a chemical reaction, mechanism depicts the actual process by which the reaction has taken place. It indicates which bonds are broken, in what order, the steps involved and the relative rate of each step. -
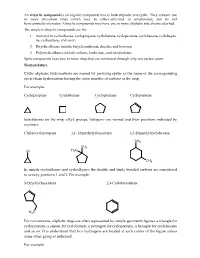
Nomenclature Cyclic Aliphatic Hydrocarbons Are Named By
An alicyclic compound is an organic compound that is both aliphatic and cyclic. They contain one or more all-carbon rings which may be either saturated or unsaturated, but do not have aromatic character. Alicyclic compounds may have one or more aliphatic side chains attached. The simplest alicyclic compounds are the 1. monocyclic cycloalkanes: cyclopropane, cyclobutane, cyclopentane, cyclohexane, cyclohepta ne, cyclooctane, and so on. 2. Bicyclic alkanes include bicycloundecane, decalin, and housane. 3. Polycyclic alkanes include cubane, basketane, and tetrahedrane. Spiro compounds have two or more rings that are connected through only one carbon atom. Nomenclature Cyclic aliphatic hydrocarbons are named by prefixing cyclo- to the name of the corresponding open-chain hydrocarbon having the same number of carbons as the ring. For example: Cyclopropane Cyclobutane Cyclopentane Cyclopentene Substituents on the ring- alkyl, groups, halogens- are named and their positions indicated by numbers. Chlorocyclopropane 1,1- Dimethylyclopentane 1,3-Dimethylcyclohexane CH3 CH3 Cl H3C CH3 In simple cycloalkenes and cycloalkynes the double and triply bonded carbons are considered to occupy positions 1 and 2. For example: 3-Ethylcyclopentene 1,3-Cyclohexadiene H3C For convenience, aliphatic rings are often represented by simple geometric figures: a triangle for cyclopropane, a square for cyclobutane, a pentagon for cyclopentane, a hexagon for cyclohexane and so on. It is understood that two hydrogens are located at each corner of the figure unless some other group is indicated. For example H3C cyclopentane 3-Ethylcyclopentene 1,3-Cyclopentadiene CH3 CH3 Cl CH Cyclohexane 3 1,3-Dimethylcyclohexane 2- Chloro-1-methylcyclohexane As usual alcohols are given the ending –ol, which takes priority over –ene and appears last in the name. -

The Smallest Quantum Vortex
“The 2D Symmetry of Nature” T.M. Lach April 11, 2005 The structures of nature and the strong nuclear force The Proton the smallest Quantum Vortex Over the years many new discoveries have taught us that nature works its miracles with very simple structures. Going back 100 years we see this simplicity in structures like the Benzene molecule, Polycyclic Aromatic Hydrocarbons (PAH), the Hydrogen atom, DNA, snowflakes and crystal lattices. (1) In recent years many new discoveries, like the discovery of Quarks, have reinforced this belief. Ninety years ago, Niels Bohr’s model of the structure of the hydrogen atom (March 6 th 1913) revolutionized science since it brought together a few simple principles that allowed us to understand the nature of why atoms have quantized energy levels. Thus began the new era of Quantum Theory and Quantum Mechanics. Many before Bohr had assumed that the electrons were circling the nucleus. (2) Nicholson earlier had assumed that the angular momentum of the electron was quantized, for 30 years others had tried to explain the Balmer series of the hydrogen spectrum, but Bohr tied it all together into a coherent picture of the hydrogen atom. Bohr had to assume that the circling electrons did not lose energy to radiation and thereby spiral into the nucleus, a big assumption at the time. Ten years later in 1923 DeBroglie proposed the wave nature of particles, which explained why the circling electrons could maintain stable quantized orbits and so began the quantum nature of the universe, with a little help from Plank, Einstein, Shrodinger, Sommerfeld and many others. -

Rodd's Chemistry of Carbon Compound S
RODD'S CHEMISTRY OF CARBON COMPOUND S A modern comprehensive treatis e SECOND EDITIO N Edited by S . COFFE Y M.Sc. (London), D.Sc. (Leyden), F .R.I.C. formerly of I.C.I. Dyestuffs Division, Blackley, Manchester VOLUME II PART C POLYCARBOCYCLIC COMPOUNDS , EXCLUDING STEROIDS PREFACE VII OFFICIAL PUBLICATIONS ; SCIENTIFIC JOURNALS AND PERIODICALS X V LIST OF COMMON ABBREVIATIONS AND SYMBOLS USED XV I Chapter 9. Polycarbocyclic Compounds with Separate Ring Systems , and Spiro Compounds N. A . J . RoGERs 1. General introduction to polycarbocyclic compounds ; classification and nomenclature a. Classification I b. Nomenclature 2 (i) Ring-systems joined directly or through a carbon chain, 3 - (ii) Spir o compounds, 3 - (iii) Fused and bridged ring-systems, 4 2 . Compounds with rings joined directly or through a carbon chain 5 a. General methods of synthesis 6 b. Polycyclopropyl compounds 6 c. Polycyclobutyl compounds 9 d. Polycyclopentyl compounds 9 e. Cyclopropylcyclopentane compounds 1 3 f. Cyclopropylcyclohexane compounds 1 3 g. Cyclobutylcyclohexane compounds 13 h. Cyclopentylcyclohexane compounds 14 i. Polycyclohexyl compounds 1 4 (i) Hydrocarbons, r4 - (ii) Hydroxy and amino derivatives, 15 - (iii) Ketones, 16 - (iv) Carboxylic acids, I q j. Cyclopentylcycloheptane compounds 1 7 k. Cyclohexylcycloheptane compounds 1 8 1. Bicycloheptyl and its derivatives 1 8 m . Bicyclo-octyl and related compounds 1 8 3. Spiro compounds ; spiranes 20 a. The spiro[z .z]pentane group 2 1 b. The spiro[2 .3]hexane group 22 c. The spiro[z .4]heptane group 23 d. The spiro[2 .5]octane group 24 e. The spiro[2,6]nonane group 2 5 f. -

Stereochemistry CHAPTER3
28 Stereochemistry CHAPTER3 Stereochemistry Stereochemistry : It involves the study of the relative spatial arrangement of atoms within the molecules. Dynamic stereochemistry: Dynamic stereochemistry is the study of the effect of stereochemistry on the rate of a chemical reaction. First Stereochemist – Louis Pasteur (1849): Significance of stereochemistry: One of the most infamous demonstration of the significance of stere- ochemistry was the Thalidomide disaster. Thalidomide is a drug was first prepared in 1957 in Germany, prescribed for treating morning Sickness in pregnant women. It was discovered that one optical isomer i.e. R- Isomer of the drug was safe whereas the S-isomer had teratogenic effect, causing serious genetic damage to early embryonic growth and development. O O H O O H N N 1 2 N 3 O N O H H 4 O R-isomer O S-isomer Drug for morning sickness in pregnant women. Teratogenic effect [Remark: In human body, Thalidomide undergoes racemization: even if only one of the two stereoisomers is ingested, the other one is produced.] Now we have another example - Propanolol. H H N N O O H OH OH H R-Propanolol (contraceptive) S-Propanolol (antihypertensive) Stereochemistry 29 SOME TERMINOLOGY Optical activity: The term optical activity derived from the interaction of chiral materials with polarized light. Scalemic: Any non-racemic chiral substance is called Scalemic. • A chiral substance is enantio pure or homochiral when only one of two possible enantiomer is present. • A chiral substance is enantio enriched or heterochiral when an excess of one enantiomer is present but not the exclusion of the other. -

Organic Chemistry Frontiers
ORGANIC CHEMISTRY FRONTIERS View Article Online REVIEW View Journal | View Issue Progress in the synthesis of perylene bisimide dyes Cite this: Org. Chem. Front., 2019, 6, Agnieszka Nowak-Król and Frank Würthner * 1272 With their versatile absorption, fluorescence, n-type semiconducting and (photo-)stability properties, per- ylene bisimides have evolved as the most investigated compounds among polycyclic aromatic hydro- carbons during the last decade. In this review we collect the results from about 200 original publications, reporting a plethora of new perylene bisimide derivatives whose properties widely enrich the possibility for the application of these dyes beyond traditional fields. While some applications are highlighted, different from other recent reviews, our focus here is on the advances in the synthetic methodologies Received 17th December 2018, that have afforded new bay functionalizations, recently addressed functionalizations at the ortho-positions Accepted 17th February 2019 to the carbonyl groups, and annulation of carbo- and heterocyclic units. An impressive number of DOI: 10.1039/c8qo01368c perylene bisimide oligomers are highlighted as well which are connected by single bonds or spiro linkage rsc.li/frontiers-organic or in a fused manner, leading to arrays with fascinating optical and electronic properties. Creative Commons Attribution 3.0 Unported Licence. Introduction are the leading examples of the class of tetrapyrrole dyes, PBIs are the most important compounds of the family of polycyclic About a hundred years after their discovery, perylene-3,4:9,10- aromatic hydrocarbons. This outstanding role of PBIs has bis(dicarboximide)s, commonly abbreviated as PDIs or PBIs, evolved not only due to their properties, but also due to the have emerged as one of the most important classes of func- incredible development of the synthetic chemistry of this class tional dyes. -

Enantiotopic Discrimination in the NMR Spectrum of Prochiral Solutes in Chiral Liquid Crystals
Chemical Society Reviews Enantiotopic Discrimination in the NMR Spectrum of Prochiral Solutes in Chiral Liquid Crystals Journal: Chemical Society Reviews Manuscript ID: CS-REV-07-2014-000260 Article Type: Review Article Date Submitted by the Author: 29-Jul-2014 Complete List of Authors: Lesot, Phillippe; Universite Paris Sud (Paris XI), LRMN, ICMMO, UMR 8182 Aroulanda, Christie; Universite Paris Sud (Paris XI), LRMN, ICMMO, UMR 8182 Zimmermann, Herbert; Abteilung Biophysik, Max-Planck-Institut für Medizinische Forschung, Luz, Zeev; Weizmann Institute of Science, Department of Chemical Physics Page 1 of 117 Chemical Society Reviews Chem. Soc. Rev. (2014) - 1 - Enantiotopic Discrimination in the NMR Spectrum of Prochiral Solutes in Chiral Liquid Crystals Philippe Lesot* ,a , Christie Aroulanda a, Herbert Zimmermann b and Zeev Luz c a Laboratoire de RMN en Milieu Orienté CNRS UMR 8182, ICMMO, Bât. 410, Université de Paris-Sud, 91405 Orsay cedex, France. bAbteilung Biophysik, Max-Planck-Institut für Medizinische Forschung, Jahnstrasse 29, 69120 Heidelberg, Germany. c Weizmann Institute of Science, Department of Chemical Physics, Rehovot 76100, Israel. Corresponding author : Philippe Lesot: [email protected] Keywords : Prochirality, Enantiotopic sites, NMR, Chiral Liquid Crystals, Dynamics. Type of article : (comprehensive) review Chemical Society Reviews Page 2 of 117 Chem. Soc. Rev. (2014) - 2 - Abstract The splitting of signals in the NMR spectra originating from enantiotopic sites in prochiral molecules when dissolved in chiral solvents is referred to as spectral enantiotopic discrimination. The phenomenon is particularly noticeable in chiral liquid crystals (CLC) due to the combined effect of the anisotropic magnetic interactions and the ordering of the solute in the mesophase. -

Copyrighted Material
Index Abbreviations receptor sites 202, 211 weak 122 amino acids, Table 500–1 muscarinic 413 weak, pH calculation 147–8 nucleic acids 551 nicotinic 413 Acid–base nucleotides, Table 551 as quaternary ammonium salt 202 catalysis, enzymes 516 peptides and proteins 504 Acetylcholinesterase equilibria 121 phosphates and diphosphates 277 enzyme mechanism 519–21 interactions, predicting 155–7 structural, Table 14 hydrolysis of acetylcholine 279 Acidic reagents, Table 157 ACE (angiotensin-converting enzyme) inhibitors 279 Acidity enzyme action 532 Acetyl-CoA carboxylase, in fatty acid acidity constant 122 inhibitors 532 biosynthesis 595 bond energy effects 125 Acetal Acetyl-CoA (acetyl coenzyme A) definition 121 in etoposide 233 as acylating agent 262 electronegativity effects 125 formation 229 carboxylation to malonyl-CoA 595, hybridization effects 128 polysaccharides as polyacetals 232 609 inductive effects 125–7 as protecting group 230 Claisen reaction 381 influence of electronic and structural Acetal and ketal enolate anions 373 features 125–34 cyclic, as protecting groups 481 in Krebs cycle 585 and leaving groups, Table 189 groups in sucrose 231 from β-oxidation of fatty acids 388 pKa values 122–5 Acetaldehyde, basicity 139 as thioester 262, 373 resonance / delocalization effects 129–34 Acetamide, basicity 139 Acetylene, bonding molecular orbitals 31 Acidity (compounds) Acetaminophen, see paracetamol Acetylenes, acidity 128 acetone 130 Acetoacetyl-CoA, biosynthesis from N-Acetylgalactosamine, in blood group acetonitrile 365 acetyl-CoA 392 -

The Control of Stereochemistry by the Pentafluorosulfanyl Group
Organic & Biomolecular Chemistry View Article Online PAPER View Journal | View Issue The control of stereochemistry by the pentafluorosulfanyl group† Cite this: Org. Biomol. Chem., 2018, 16, 3151 Paul R. Savoie, Cortney N. von Hahmann, Alexander Penger, Zheng Wei and John T. Welch * The influence of pentafluorosulfanylation on biological activity has been revealed in numerous compara- tive studies of biologically active compounds, but considerably less is known about the influence of pen- tafluorosulfanylation on reactivity. Among the distinctive properties of the pentafluorosulfanyl group is the profound dipole moment that results from introduction of this substituent. It has been shown that dipolar Received 20th December 2017, effects coupled with the steric demand of the SF5 group may be employed to influence the stereo- Accepted 3rd April 2018 chemistry of reactions, especially those processes with significant charge separation in the transition DOI: 10.1039/c7ob03146g state. The Staudinger ketene-imine cycloaddition reaction is an ideal platform for investigation of dipolar rsc.li/obc control of diastereoselectivity by the pentafluorosulfanyl group. Introduction ation into a hydrocarbon chain, the restricted rotation about the carbon–sulfur bond that results from interactions with Numerous pentafluorosulfanyl(SF5)-containing organic nearby methylene groups, can lead to localized conformational – compounds1 10 have been prepared that have potential utility rigidity of the alkyl chain.21,22 in drug discovery, agrochemical synthesis and materials -

Introduction to Organic Chemistry 2018 More
Introduction to Organic Chemistry 25 Introduction to Organic Chemistry Handout 2 - Stereochemistry OH O O OH enantiomers Me OH HO Me A B NH2 NH2 diastereomers diastereomers diastereomers OH O O OH Me OH HO Me C D NH2 enantiomers NH2 http://burton.chem.ox.ac.uk/teaching.html ◼ Organic Chemistry J. Clayden, N. Greeves, S. Warren ◼ Stereochemistry at a Glance J. Eames & J. M. Peach ◼ The majority of organic chemistry text books have good chapters on the topics covered by these lectures ◼ Eliel Stereochemistry of Organic Compounds (advanced reference text) Introduction to Organic Chemistry 26 ◼ representations of formulae in organic chemistry ◼ skeletal representations are far less cluttered and as a result are much clearer than drawing all carbon and hydrogen atoms explicitly, they also give a much better representation of the likely bond angles and hence hybridisation states of the carbon atoms ◼ skeletal representations allow functional groups (sites of reactivity) to be clearly seen ◼ guidelines for drawing skeletal structures i) draw chains of atoms as zig-zags ii) do not draw C atoms unless there is good reason to draw them iii) do not draw C-H bonds unless there is good reason to draw then iv) do not draw Hs attached to carbon atoms unless there is good reason to draw them v) make drawings realistic Introduction to Organic Chemistry 27 ◼ representing structures in three dimensions ◼ a wedged bond indicates the bond is projecting out in front of the plane of the paper ◼ a dashed bond indicates the bond is projecting behind the plane -

By M. TICHY Institute of Organic Chemistry and Biochemistry, Czechoslovak Academy of Sciences, Prague (Received October 10, 1975)
CHEMISTRY OF TWISTANE SYSTEM AND ITS USE IN STEREOCHEMISTRY* By M. TICHY Institute of Organic Chemistry and Biochemistry, Czechoslovak Academy of Sciences, Prague (Received October 10, 1975) The aim of the present review is to show the chemistry of twistane system and the various ways in which it can be used as a stereochemical model of great utility. Many of the stereochemical appli- cations mentioned are the result of investigations made in the author's Laboratory. Twistane — tricyclo(4,4,0,03,8)decane (I) — belongs to the great family of adamantane isomers** of the formula C10H16. It is composed solely of six-membered rings, twisted in the same sense. The parent hydrocarbon has three two-fold rota- tional axes of symmetry (D2) and exists in two enantiomeric forms which differ in the sense of twist: according to the CAHN—INGOLD—PRELOG nomenclature [11] the enantiomer (la) has P-helicity whereas the other (lb) M-helicity. la lb • * This review is based on a lecture presented at A. Jôzsef University, Szeged, and on the review in Chem. Listy 69, 45 (1975). »* For reviews on adamantane chemistry see [1—3], adamantane isomers other than twistane are studied e.g. in ref. [4—10]. 158 M. TICHY There are three groups of sterically analogous carbon atoms in this system: carbons 1,3,6,8, carbons 4,5,9,10 and carbons 2 and 7. Whereas the two bonds at C(2) and C(7) in the parent hydrocarbon are sterically equivalent, the bonds in positions 4,5,9 and 10 are non-equivalent and therefore substitution in these positions affords two diastereoisomeric (monosubstituted) derivatives (Formulae A and B).