THE ELECTROLYTIC PRODUCTION of PEROXYDISULFURIC ACID It USING PERIODICALLY REVERSED DIRECT CURRENT and ALTERNATING CURRENT SUPERIMPOSED on DIRECT CURRENT
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

4월 24일(목), 09:00~10:30
포스터 I: 4월 24일(목), 09:00~10:30 공업화학: 4월 24일(목), 09:00 ~ 10:30 좌장: 박정훈(동국대) Cleaning system study with mounting activated species of solution using P공업목-1(118) (강원대)권흥수, 이원규 atmospheric pressure plasma 기공구조 조절 제올라이트 촉매를 이용한 액체연료의 흡열량 향상 (고려대)현동훈, 김중연, 김유리, 전병희, P공업목-2(118) (우수 포스터 발표상 후보) 김성현, (국방과학(연))정병훈, 한정식 (고려대)신동건, 김성현, 이창훈, P공업목-3(118) 열 안정성이 우수한 CO 흡착제용 다공성 유무기 복합 지지체 제조 2 조동현, 정현철 (고려대)김유리, 김성현, 전병희, P공업목-4(119) 분산제의 구조에 따른 Jet A-1 연료의 열산화반응 탄소침적물 감소 연구 김중연, 현동훈 (국방과학(연))한정식, 정병훈 Effect of SAA Pretreatment on Temperature of Enzymatic Hydrolysis Using P공업목-5(119) (경기대)장서윤, 박장한, 김준석 Lignocellulosic Biomass Pretreatment and fractionation of Helianthus tuberosus residue by flow-through P공업목-6(119) (경기대)박용철, 박장한, 김준석 reaction (순천대)오성준 (순천대)양지선, 박권필 P공업목-7(119) Carbon Felt 산처리에 의한 바나듐 레독스 흐름전지에 대한 영향 ((주)CNL Energy)나일채, 이정훈 ((주)ETIS)추천호 메틸카바메이트 및 메탄올을 이용한 디메틸카보네이트 제조를 위한 고활성 P공업목-8(120) (한양대)이재홍, 서영웅 촉매 조사 (우수 포스터 발표상 후보) 연속흐름반응기에서 고농도 NaBH 가수분해 반응의 촉매 종류에 따른 P공업목-9(120) 4 (순천대)오성준, 정현승, 박권필 수소발생연구 (순천대)안명원, 곽동영, 복영빈, 박권필 P공업목-10(120) 해조류 종류별 후코산틴 추출 및 염분세척 ((주) ETIS)추천호, 김영숙 (순천대)이세훈, 박권필, ((주)ETIS)김영숙, P공업목-11(120) 효소연료전지에서 Anode 조건에 따른 OCV 변화 추천호, ((주)CNL Energy)나일채 (순천대)정재현, 정회범, 박권필 P공업목-12(121) PEMFC MEA제법에 의한 성능 및 내구성 향상 ((주)CNL Energy)라일채, 이 호 (순천대)정재현, 신은경, 정회범, 박권필 P공업목-13(121) PEM 수전해 과정에서 전극과 전해질 막의 변화 ((주)CNL Energy)라일채, 이 호 (순천대)정재현, 박권필 P공업목-14(121) 직접 개미산 연료전지(DFAFC)의 구동 조건에 따른 성능 비교 ((주)CNL Energy)나일채 (순천대)정재진, 박권필 P공업목-15(121) 가속화도 4.0의 전극, 막, MEA 가속화 기법 개발 (현대자동차)안병기, 김세훈, 고재준 (순천대)정재진, 박권필 P공업목-16(122) 탄화수소막을 사용한 -

Introduction to the Preparation and Properties of Hydiogen Peroxide
CHAPTER 1 Introduction to the Preparation and Properties of Hydiogen Peroxide 1 Introduction The following chapter will discuss the preparation of hydrogen peroxide, historically, the present day and future vistas for its in situ preparation. A brief introduction to the physical properties of hydrogen peroxide will also be made for the sake of completeness. Finally, the chapter will conclude with a practical approach to the safe handling of peroxygen species, destruction of residual peroxygens, and the toxicological and occupational health considerations required when handling hydrogen peroxide. 2 Industrial Manufacture of Hydrogen Peroxide The industrial manufacture of hydrogen peroxide can be traced back to its isolation in 18 18 by L. J. Thenard. Thenard reacted barium peroxide with nitric acid to produce a low concentration of aqueous hydrogen peroxide; the process can, however, be significantly improved by the use of hydrochloric acid. The hydrogen peroxide is formed in conjunction with barium chloride, both of which are soluble in water. The barium chloride is subsequently removed by precipitation with sulfuric acid (Figure 1.1). Hence, Thenard gave birth to the first commercial manufacture of aqueous hydrogen peroxide, although it took over sixty years before Thenard’s wet chemical process was employed in a commercial capacity.2 The industrial production of hydrogen peroxide using the above route was still operating until the middle of the 20th century. At the turn of the 19th century, approximately 10000 metric tonnes per annurn of barium peroxide were converted to about 2000 metric tonnes of hydrogen peroxide. Thenard’s process has, however, some major drawbacks which quenched the expectant explosion of its use in an aqueous form. -

Preparing to Manufacture Hydrogen Peroxide
PREPARING TO MANUFACTURE HYDROGEN PEROXIDE Part of the Hydrogen Peroxide Propulsion Guide The early laboratory preparation of hydrogen peroxide was based on the technique that Thenard used during the initial preparation of hydrogen peroxide. In this technique, barium nitrate, purified by recrystallization, was decomposed by heating in air in a porcelain retort. The resulting oxide was further oxidized by heating in a stream of oxygen to a dull red heat. The barium peroxide which formed was then dampened, ground, and dissolved in hydrochloric acid (nitric acid was used in Thenard’s initial experiments). A slight excess of sulfuric acid was then added to precipitate barium sulfate and regenerate hydrochloric acid. The procedure of barium peroxide solution and sulfate precipitation was repeated several times in the same solution to increase the peroxide concentration (concentrations of up to 33 percent by weight hydrogen peroxide could be achieved in this manner). The concentrated solution containing water, hydrogen peroxide, and hydrochloric acid, along with accumulated impurities, was cooled with ice and saturated with barium peroxide; iron and manganese impurities in the solution were then precipitated out as phosphates. The hydrochloric acid was removed by the addition of silver sulfate and the sulfate ion was removed by the subsequent addition of barium oxide. Further concentration was accomplished by vacuum distillation until “no further density increase occurs.” Thenard reported that 100 w/o hydrogen peroxide (on the basis of density data and the measurement of the volume of oxygen released) could be obtained by this technique. The first record of commercial production of hydrogen peroxide appeared in the 1865 to 1875 period. -

Periodic Trends in the Main Group Elements
Chemistry of The Main Group Elements 1. Hydrogen Hydrogen is the most abundant element in the universe, but it accounts for less than 1% (by mass) in the Earth’s crust. It is the third most abundant element in the living system. There are three naturally occurring isotopes of hydrogen: hydrogen (1H) - the most abundant isotope, deuterium (2H), and tritium 3 ( H) which is radioactive. Most of hydrogen occurs as H2O, hydrocarbon, and biological compounds. Hydrogen is a colorless gas with m.p. = -259oC (14 K) and b.p. = -253oC (20 K). Hydrogen is placed in Group 1A (1), together with alkali metals, because of its single electron in the valence shell and its common oxidation state of +1. However, it is physically and chemically different from any of the alkali metals. Hydrogen reacts with reactive metals (such as those of Group 1A and 2A) to for metal hydrides, where hydrogen is the anion with a “-1” charge. Because of this hydrogen may also be placed in Group 7A (17) together with the halogens. Like other nonmetals, hydrogen has a relatively high ionization energy (I.E. = 1311 kJ/mol), and its electronegativity is 2.1 (twice as high as those of alkali metals). Reactions of Hydrogen with Reactive Metals to form Salt like Hydrides Hydrogen reacts with reactive metals to form ionic (salt like) hydrides: 2Li(s) + H2(g) 2LiH(s); Ca(s) + H2(g) CaH2(s); The hydrides are very reactive and act as a strong base. It reacts violently with water to produce hydrogen gas: NaH(s) + H2O(l) NaOH(aq) + H2(g); It is also a strong reducing agent and is used to reduce TiCl4 to titanium metal: TiCl4(l) + 4LiH(s) Ti(s) + 4LiCl(s) + 2H2(g) Reactions of Hydrogen with Nonmetals Hydrogen reacts with nonmetals to form covalent compounds such as HF, HCl, HBr, HI, H2O, H2S, NH3, CH4, and other organic and biological compounds. -

Download Author Version (PDF)
PCCP Accepted Manuscript This is an Accepted Manuscript, which has been through the Royal Society of Chemistry peer review process and has been accepted for publication. Accepted Manuscripts are published online shortly after acceptance, before technical editing, formatting and proof reading. Using this free service, authors can make their results available to the community, in citable form, before we publish the edited article. We will replace this Accepted Manuscript with the edited and formatted Advance Article as soon as it is available. You can find more information about Accepted Manuscripts in the Information for Authors. Please note that technical editing may introduce minor changes to the text and/or graphics, which may alter content. The journal’s standard Terms & Conditions and the Ethical guidelines still apply. In no event shall the Royal Society of Chemistry be held responsible for any errors or omissions in this Accepted Manuscript or any consequences arising from the use of any information it contains. www.rsc.org/pccp Page 1 of 24 Physical Chemistry Chemical Physics Disulfuric acid dissociated by two water molecules: Ab initio and density functional theory calculations Seong Kyu Kim, 1,* Han Myoung Lee, 2 Kwang S. Kim 2,* 1 Department of Chemistry, Sungkyunkwan University, Suwon 440-746, Korea Manuscript 2Center for Superfunctional Materials, Department of Chemistry, Ulsan Institute of Science and Technology (UNIST), Ulsan 689-798, Korea *Corresponding authors, [email protected] , [email protected] Accepted Abstract We have studied geometries, energies and vibrational spectra of disulfuric acid - (H 2S2O7) and its anion (HS 2O7 ) hydrated by a few water molecules, using density functional theory (M062X) and ab initio theory (SCS-MP2 and CCSD(T)). -

Most Common Substances in Chemicals for Different Industries
Table 14 Most common substances by industry Figures for 2009. Substances contained in more than 10 products. A substance can be omitted for secrecy reasons. Industry CAS-no Substance Number of products A01 Agriculture 7732-18-5 Water 458 6484-52-2 Nitric acid ammonium salt 77 57-55-6 1,2-Propanediol 75 64742-65-0 Distillates (petroleum), solvent-dewaxed heavy 74 paraffinic 63148-62-9 Siloxanes and Silicones, di-Me 60 57-13-6 Urea 54 67-63-0 2-Propanol 54 11138-66-2 Xanthan gum 53 7783-20-2 Sulfuric acid diammonium salt 53 7722-76-1 Phosphoric acid, monoammonium salt 52 7487-88-9 Sulfuric acid magnesium salt (1:1) 51 7447-40-7 Potassium chloride 46 56-81-5 1,2,3-Propanetriol 44 7778-80-5 Sulfuric acid dipotassium salt 43 1310-73-2 Sodium hydroxide 41 7647-14-5 Sodium chloride 41 2634-33-5 1,2-Benzisothiazol-3(2H)-one 38 1310-58-3 Potassium hydroxide 33 7757-79-1 Nitric acid potassium salt 33 7785-87-7 Manganese sulfate 32 7664-38-2 Phosphoric acid 32 72623-87-1 Lubricating oils, petroleum, C20-50, 31 hydrotreated neutral oil-based 64741-89-5 Distillates (petroleum), solvent-refined light 29 paraffinic 77-92-9 1,2,3-Propanetricarboxylic acid, 2-hydroxy- 28 8061-51-6 Lignosulfonic acid, sodium salt 28 7778-18-9 Sulfuric acid, calcium salt (1:1) 27 64-17-5 Ethanol 27 14807-96-6 Talc 26 64742-94-5 Solvent naphtha (petroleum), heavy arom. 25 68649-42-3 Phosphorodithioic acid, O,O-di-C1-14-alkyl 24 esters, zinc salts (2:1) 64-02-8 Glycine, N,N'-1,2-ethanediylbis[N- 24 (carboxymethyl)-, tetrasodium salt 64-19-7 Acetic acid 23 99-76-3 Benzoic acid, -
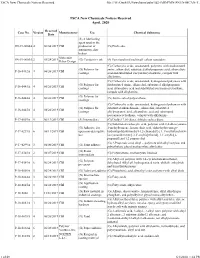
TSCA New Chemicals Notices Received File:///W:/Oneepa/Newchems/Pubs/5D2-IMD/PMN-SNUN-MCAN-T
TSCA New Chemicals Notices Received file:///W:/OneEPA/Newchems/pubs/5d2-IMD/PMN-SNUN-MCAN-T... TSCA New Chemicals Notices Received April, 2020 Received Case No. Version Manufacturer Use Chemical Substance Date (S) A lubricating agent used in the SN-19-0004A 4 06/04/2019 CBI production of (G) Pitch coke automotive disc brakes Molecular SN-19-0005A 2 05/28/2019 (G) Conductive ink (S) Functionalized multiwall carbon nanotubes Rebar Design (G) Carboxylic acids, unsaturated, polymers with disubstituted (G) Polymer for amine, alkanediol, substituted alkylpropanoic acid, alkanedioic P-16-0442A 4 06/26/2019 CBI coatings acid and substituted isocyanatocycloalkane, compds with alkylamine (G) Carboxylic acids, unsaturated, hydrogenated polymers with (G) Polymer for disubstituted amine, alkanediol, substituted alkylpropanoic P-16-0443A 4 06/26/2019 CBI coatings acid, alkanedioic acid and substituted isocyanatocycloalkane, compds with alkylamine (G) Polymer for P-16-0444A 4 06/26/2019 CBI (G) Amine salted polyurethane coatings (G) Carboxylic acids, unsaturated, hydrogenated polymers with (G) Polymer for substituted alkanediamine, alkanediol, substituted P-16-0445A 4 06/26/2019 CBI coatings alkylpropanoic acid, alkanedioic acid and substituted isocyanatocycloalkane, compds with alkylamine P-17-0007A 5 06/13/2019 CBI (S) Intermediate (G) Dialkyl 7,10-dioxa, dithiahexadeca diene (G) Substituted carboxylic acid, polymer with 2,4-diisocyanato- (G) Adhesive for 1-methylbenzene, hexanedioic acid, alpha-hydro-omega- P-17-0239A 6 06/11/2019 CBI open non-descriptive -

Sulfur Dioxide in Workplace Atmospheres (Bubbler)
Withdrawn Provided For Historical Reference Only SULFUR DIOXIDE IN WORKPLACE ATMOSPHERES (BUBBLER) ♦ Method Number: ID-104 Matrix: Air OSHA PEL Sulfur Dioxide (Final Rule Limit): 2 ppm (Time Weighted Limit) 5 ppm (Short-Term Exposure Limit) Sulfur Dioxide (Transitional Limit): 5 ppm (Time Weighted Limit) Collection Device: A calibrated personal sampling pump is used to draw a known volume of air through a midget-fritted glass bubbler containing 10 to 15 mL of 0.3 N hydrogen peroxide. Recommended Air Volume: 15 to 60 L Recommended Sampling Rate: 1 L/min Analytical Procedure: Samples are directly analyzed with no sample preparation by ion chromatography as total sulfate. Detection Limit Qualitative: 0.0041 ppm (60-L air volume) Quantitative: 0.010 ppm (60-L air volume) Precision and Accuracy Validation Level: 2.5 to 10.0 ppm (60-L air volume) CVT 0.012 Bias -0.046 Overall Error ±7% Method Classification: Validated Method Chemist: Ted Wilczek, Edward Zimowski Date (Date Revised): 1981 (December, 1989) Commercial manufacturers and products mentioned in this method are for descriptive use only and do not constitute endorsements by USDOL-OSHA. Similar products from other sources can be substituted. Branch of Inorganic Methods Development OSHA Technical Center Salt Lake City, Utah 1 of 9 Note: OSHA no longer uses or supports this method (November 2019). Withdrawn Provided For Historical Reference Only 1. Introduction This method describes the collection and analysis of airborne sulfur dioxide (SO2) using midget-fritted glass bubblers (MFGBs) in the workplace. It is applicable for both short-term (STEL) and time weighted average (TWA) exposure evaluations. -

Guidelines for the Preservation of Raw Milk by Use of the Lactoperoxidase System
GUIDELINES FOR THE PRESERVATION OF RAW MILK BY USE OF THE LACTOPEROXIDASE SYSTEM CAC/GL 13-1991 INTRODUCTION Milk is an easily perishable raw material. Contaminating bacteria may multiply rapidly and render it unsuitable for processing and/or unfit for human consumption. Bacterial growth can be retarded by refrigeration, thereby slowing down the rate of deterioration. Under certain conditions refrigeration may not be feasible due to economical and/ or technical reasons. Difficulties in applying refrigeration are specially a problem for certain areas in countries setting up or expanding their milk production. In these situations, it would be beneficial to have access to a method, other than refrigeration, for retarding bacterial growth in raw milk during collection and transportation to the dairy processing plant. In 1967 the FAO/WHO Expert Panel on Milk Quality concluded that the use of hydrogen peroxide might be an acceptable alternative in the early stages of development of an organized dairy industry, provided that certain conditions were complied with. However, this method has not achieved any general acceptance as it has several drawbacks, most important of which is the difficulty of controlling its use: it may be misused to disguise milk of basic hygienic quality produced under poor hygienic conditions. The toxicological aspects of the use of relatively high concentrations of hydrogen peroxide in milk have also been questioned. A chemical method for preserving milk would still be of great advantage in certain situations. The search for such a method has therefore continued. Interest has recently been focused on the indigenous antibacterial systems in milk to determine if these could be applied practically to preserve raw milk. -

Chemical Names and CAS Numbers Final
Chemical Abstract Chemical Formula Chemical Name Service (CAS) Number C3H8O 1‐propanol C4H7BrO2 2‐bromobutyric acid 80‐58‐0 GeH3COOH 2‐germaacetic acid C4H10 2‐methylpropane 75‐28‐5 C3H8O 2‐propanol 67‐63‐0 C6H10O3 4‐acetylbutyric acid 448671 C4H7BrO2 4‐bromobutyric acid 2623‐87‐2 CH3CHO acetaldehyde CH3CONH2 acetamide C8H9NO2 acetaminophen 103‐90‐2 − C2H3O2 acetate ion − CH3COO acetate ion C2H4O2 acetic acid 64‐19‐7 CH3COOH acetic acid (CH3)2CO acetone CH3COCl acetyl chloride C2H2 acetylene 74‐86‐2 HCCH acetylene C9H8O4 acetylsalicylic acid 50‐78‐2 H2C(CH)CN acrylonitrile C3H7NO2 Ala C3H7NO2 alanine 56‐41‐7 NaAlSi3O3 albite AlSb aluminium antimonide 25152‐52‐7 AlAs aluminium arsenide 22831‐42‐1 AlBO2 aluminium borate 61279‐70‐7 AlBO aluminium boron oxide 12041‐48‐4 AlBr3 aluminium bromide 7727‐15‐3 AlBr3•6H2O aluminium bromide hexahydrate 2149397 AlCl4Cs aluminium caesium tetrachloride 17992‐03‐9 AlCl3 aluminium chloride (anhydrous) 7446‐70‐0 AlCl3•6H2O aluminium chloride hexahydrate 7784‐13‐6 AlClO aluminium chloride oxide 13596‐11‐7 AlB2 aluminium diboride 12041‐50‐8 AlF2 aluminium difluoride 13569‐23‐8 AlF2O aluminium difluoride oxide 38344‐66‐0 AlB12 aluminium dodecaboride 12041‐54‐2 Al2F6 aluminium fluoride 17949‐86‐9 AlF3 aluminium fluoride 7784‐18‐1 Al(CHO2)3 aluminium formate 7360‐53‐4 1 of 75 Chemical Abstract Chemical Formula Chemical Name Service (CAS) Number Al(OH)3 aluminium hydroxide 21645‐51‐2 Al2I6 aluminium iodide 18898‐35‐6 AlI3 aluminium iodide 7784‐23‐8 AlBr aluminium monobromide 22359‐97‐3 AlCl aluminium monochloride -

SAFETY DATA SHEET Sodium Persulfate
SAFETY DATA SHEET Sodium Persulfate SDS # : 7775-27-1 Revision date: 2021-02-17 Format: NA Version 1.06 1. PRODUCT AND COMPANY IDENTIFICATION Product Identifier Product Name Sodium Persulfate CAS-No 7775-27-1 Synonyms Sodium Peroxydisulfate; Disodium Peroxydisulfate; Peroxydisulfuric acid, disodium salt; Peroxydisulfuric acid, sodium salt. Recommended use of the chemical and restrictions on use Recommended Use: Polymerization initiator; Etchant and cleaner for printed circuit boards; Hair bleaching formulations; Secondary oil recovery; Oxidizing agent for a variety of organic reactions. Restrictions on Use No uses to be advised against were identified. Manufacturer/Supplier PeroxyChem LLC 2005 Market Street Suite 3200 Philadelphia, PA 19103 Phone: +1 267/ 422-2400 (General Information) E-Mail: [email protected] Emergency telephone numbers For leak, fire, spill or accident emergencies, call: 1 800 / 424 9300 (CHEMTREC - U.S.A.) 1 703 / 527 3887 (CHEMTREC - Collect - All Other Countries) +1 303/ 389-1409 (Medical - U.S. - Call Collect) Page 1 / 10 Sodium Persulfate SDS # : 7775-27-1 Revision date: 2021-02-17 Version 1.06 2. HAZARDS IDENTIFICATION Classification OSHA Regulatory Status This material is considered hazardous by the OSHA Hazard Communication Standard (29 CFR 1910.1200) Acute toxicity - Oral Category 4 Skin corrosion/irritation Category 2 Serious eye damage/eye irritation Category 2B Respiratory sensitization Category 1 Skin sensitization Category 1 Specific target organ toxicity (single exposure) Category 3 Oxidizing Solids -

2 1 0 Material Safety Data Sheet
He a lt h 1 2 Fire 2 1 0 Re a c t iv it y 0 Pe rs o n a l Pro t e c t io n H Material Safety Data Sheet Methylene Blue Solution, Loeffler MSDS Section 1: Chemical Product and Company Identification Product Name: Methylene Blue Solution, Loeffler Contact Information: Catalog Codes: SLM3328 Sciencelab.com, Inc. 14025 Smith Rd. CAS#: Mixture. Houston, Texas 77396 RTECS: Not applicable. US Sales: 1-800-901-7247 International Sales: 1-281-441-4400 TSCA: TSCA 8(b) inventory: Potassium hydroxide; Ethyl alcohol 200 Proof; Water Order Online: ScienceLab.com CI#: Not applicable. CHEMTREC (24HR Emergency Telephone), call: 1-800-424-9300 Synonym: Methylene Blue-Loeffler's Alkaline International CHEMTREC, call: 1-703-527-3887 Chemical Name: Not applicable. For non-emergency assistance, call: 1-281-441-4400 Chemical Formula: Not applicable. Section 2: Composition and Information on Ingredients Composition: Name CAS # % by Weight Potassium hydroxide 1310-58-3 <1 Ethyl alcohol 200 Proof 64-17-5 25-30 Water 7732-18-5 70-75 Methylene Blue CI52015 7220-79-3 <1 Toxicological Data on Ingredients: Ethyl alcohol 200 Proof: ORAL (LD50): Acute: 7060 mg/kg [Rat.]. 3450 mg/kg [Mouse]. VAPOR (LC50): Acute: 20000 ppm 8 hours [Rat]. 39000 mg/m 4 hours [Mouse]. Section 3: Hazards Identification Potential Acute Health Effects: Hazardous in case of skin contact (irritant, permeator), of eye contact (irritant), of ingestion. Potential Chronic Health Effects: CARCINOGENIC EFFECTS: Classified PROVEN by State of California Proposition 65 [Ethyl alcohol 200 Proof]. Classified A4 (Not classifiable for human or animal.) by ACGIH [Ethyl alcohol 200 Proof].