Anderson2009chap22.Pdf
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Leaf Anatomy and C02 Recycling During Crassulacean Acid Metabolism in Twelve Epiphytic Species of Tillandsia (Bromeliaceae)
Int. J. Plant Sci. 154(1): 100-106. 1993. © 1993 by The University of Chicago. All rights reserved. 1058-5893/93/5401 -0010502.00 LEAF ANATOMY AND C02 RECYCLING DURING CRASSULACEAN ACID METABOLISM IN TWELVE EPIPHYTIC SPECIES OF TILLANDSIA (BROMELIACEAE) VALERIE S. LOESCHEN,* CRAIG E. MARTIN,' * MARIAN SMITH,t AND SUZANNE L. EDERf •Department of Botany, University of Kansas, Lawrence, Kansas 66045-2106; and t Department of Biological Sciences, Southern Illinois University, Edwardsville, Illinois 62026-1651 The relationship between leaf anatomy, specifically the percent of leaf volume occupied by water- storage parenchyma (hydrenchyma), and the contribution of respiratory C02 during Crassulacean acid metabolism (CAM) was investigated in 12 epiphytic species of Tillandsia. It has been postulated that the hydrenchyma, which contributes to C02 exchange through respiration only, may be causally related to the recently observed phenomenon of C02 recycling during CAM. Among the 12 species of Tillandsia, leaves of T. usneoides and T. bergeri exhibited 0% hydrenchyma, while the hydrenchyma in the other species ranged from 2.9% to 53% of leaf cross-sectional area. Diurnal malate fluctuation and nighttime atmospheric C02 uptake were measured in at least four individuals of each species. A significant excess of diurnal malate fluctuation as compared with atmospheric C02 absorbed overnight was observed only in T. schiedeana. This species had an intermediate proportion (30%) of hydrenchyma in its leaves. Results of this study do not support the hypothesis that C02 recycling during CAM may reflect respiratory contributions of C02 from the tissue hydrenchyma. Introduction tions continue through fixation of internally re• leased, respired C02 (Szarek et al. -

Partial Flora Survey Rottnest Island Golf Course
PARTIAL FLORA SURVEY ROTTNEST ISLAND GOLF COURSE Prepared by Marion Timms Commencing 1 st Fairway travelling to 2 nd – 11 th left hand side Family Botanical Name Common Name Mimosaceae Acacia rostellifera Summer scented wattle Dasypogonaceae Acanthocarpus preissii Prickle lily Apocynaceae Alyxia Buxifolia Dysentry bush Casuarinacea Casuarina obesa Swamp sheoak Cupressaceae Callitris preissii Rottnest Is. Pine Chenopodiaceae Halosarcia indica supsp. Bidens Chenopodiaceae Sarcocornia blackiana Samphire Chenopodiaceae Threlkeldia diffusa Coast bonefruit Chenopodiaceae Sarcocornia quinqueflora Beaded samphire Chenopodiaceae Suada australis Seablite Chenopodiaceae Atriplex isatidea Coast saltbush Poaceae Sporabolis virginicus Marine couch Myrtaceae Melaleuca lanceolata Rottnest Is. Teatree Pittosporaceae Pittosporum phylliraeoides Weeping pittosporum Poaceae Stipa flavescens Tussock grass 2nd – 11 th Fairway Family Botanical Name Common Name Chenopodiaceae Sarcocornia quinqueflora Beaded samphire Chenopodiaceae Atriplex isatidea Coast saltbush Cyperaceae Gahnia trifida Coast sword sedge Pittosporaceae Pittosporum phyliraeoides Weeping pittosporum Myrtaceae Melaleuca lanceolata Rottnest Is. Teatree Chenopodiaceae Sarcocornia blackiana Samphire Central drainage wetland commencing at Vietnam sign Family Botanical Name Common Name Chenopodiaceae Halosarcia halecnomoides Chenopodiaceae Sarcocornia quinqueflora Beaded samphire Chenopodiaceae Sarcocornia blackiana Samphire Poaceae Sporobolis virginicus Cyperaceae Gahnia Trifida Coast sword sedge -

Restoration After Removal of Pines at Gnangara Final
RESTORATION OF BANKSIA WOODLAND AFTER THE REMOVAL OF PINES AT GNANGARA: SEED SPECIES REQUIREMENTS AND PRESCRIPTIONS FOR RESTORATION A report prepared on behalf of the Department of Environment and Conservation for the Gnangara Sustainability Strategy Kellie Maher University of Western Australia May 2009 Restoration of Banksia woodland after the removal of pines at Gnangara: seed species requirements and prescriptions for restoration Report for the Department of Environment and Conservation Kellie Maher University of Western Australia Gnangara Sustainability Strategy Taskforce Department of Water 168 St Georges Terrace Perth Western Australia 6000 Telephone +61 8 6364 7600 Facsimile +61 8 6364 7601 www.gnangara.water.wa.gov.au © Government of Western Australia 2009 May 2009 This work is copyright. You may download, display, print and reproduce this material in unaltered form only (retaining this notice) for your personal, non-commercial use or use within your organisation. Apart from any use as permitted under the Copyright Act 1968 , all other rights are reserved. Requests and inquiries concerning reproduction and rights should be addressed to the Department of Conservation and Environment. This document has been commissioned/produced as part of the Gnangara Sustainability Strategy (GSS). The GSS is a State Government initiative which aims to provide a framework for a whole of government approach to address land use and water planning issues associated with the Gnangara groundwater system. For more information go to www.gnangara.water.wa.gov.au 1 Restoration of Banksia woodland after the removal of pines at Gnangara: seed species requirements and prescriptions for restoration A report to the Department of Environment and Conservation Kellie Maher University of Western Australia May 2009 2 Table of Contents List of Tables .................................................................................................................... -

Spanish Moss and Ball Moss 1
FOR52 Spanish Moss and Ball Moss 1 Nancy P. Arny2 Spanish moss (Tillandsia usneoides) and ball Bromeliads moss (T. recurvata) are common elements of the Florida landscape. They are two of Florida's native Like almost all members of the Bromeliaceae, members of the Bromeliaceae, also known as the Spanish moss and ball moss are perennial herbs. This pineapple family. This family includes species as means they do not have permanent woody stems diverse as pineapples, Spanish moss and a above ground, but that individual plants persist for carnivorous relative native to Australia. Bromeliads years and will reproduce without human intervention. are members of the plant division Like many other bromeliads, these plants are Magnoliophyta--the flowering plants. While most epiphytes or "air plants". This indicates that they do Floridians are at least vaguely familiar with Spanish not require soil to root in, but can survive and thrive moss, many have never seen it flower and may be growing above the ground hanging on branches of surprised at the beauty of its delicate blossom. Of trees or other structures. They are not parasites. course, the fact that both Spanish moss and ball moss Without soil as a source of nutrients, these plants produce flowers is proof that they are not truly have evolved the capacity to make use of minerals mosses at all. dissolved in the water which flows across leaves and down branches. This fact sheet will help the reader to distinguish between the two common Tillandsias . It also Spanish moss plants appear to vary in mineral provides basic information on the biology and content and it has been proven that they gain a ecology of these fascinating plants and provides significant portion of their nutrients from stem recommendations for their management in the home run-off from the trees on which they are anchored. -

Outline of Angiosperm Phylogeny
Outline of angiosperm phylogeny: orders, families, and representative genera with emphasis on Oregon native plants Priscilla Spears December 2013 The following listing gives an introduction to the phylogenetic classification of the flowering plants that has emerged in recent decades, and which is based on nucleic acid sequences as well as morphological and developmental data. This listing emphasizes temperate families of the Northern Hemisphere and is meant as an overview with examples of Oregon native plants. It includes many exotic genera that are grown in Oregon as ornamentals plus other plants of interest worldwide. The genera that are Oregon natives are printed in a blue font. Genera that are exotics are shown in black, however genera in blue may also contain non-native species. Names separated by a slash are alternatives or else the nomenclature is in flux. When several genera have the same common name, the names are separated by commas. The order of the family names is from the linear listing of families in the APG III report. For further information, see the references on the last page. Basal Angiosperms (ANITA grade) Amborellales Amborellaceae, sole family, the earliest branch of flowering plants, a shrub native to New Caledonia – Amborella Nymphaeales Hydatellaceae – aquatics from Australasia, previously classified as a grass Cabombaceae (water shield – Brasenia, fanwort – Cabomba) Nymphaeaceae (water lilies – Nymphaea; pond lilies – Nuphar) Austrobaileyales Schisandraceae (wild sarsaparilla, star vine – Schisandra; Japanese -

Alphabetical Lists of the Vascular Plant Families with Their Phylogenetic
Colligo 2 (1) : 3-10 BOTANIQUE Alphabetical lists of the vascular plant families with their phylogenetic classification numbers Listes alphabétiques des familles de plantes vasculaires avec leurs numéros de classement phylogénétique FRÉDÉRIC DANET* *Mairie de Lyon, Espaces verts, Jardin botanique, Herbier, 69205 Lyon cedex 01, France - [email protected] Citation : Danet F., 2019. Alphabetical lists of the vascular plant families with their phylogenetic classification numbers. Colligo, 2(1) : 3- 10. https://perma.cc/2WFD-A2A7 KEY-WORDS Angiosperms family arrangement Summary: This paper provides, for herbarium cura- Gymnosperms Classification tors, the alphabetical lists of the recognized families Pteridophytes APG system in pteridophytes, gymnosperms and angiosperms Ferns PPG system with their phylogenetic classification numbers. Lycophytes phylogeny Herbarium MOTS-CLÉS Angiospermes rangement des familles Résumé : Cet article produit, pour les conservateurs Gymnospermes Classification d’herbier, les listes alphabétiques des familles recon- Ptéridophytes système APG nues pour les ptéridophytes, les gymnospermes et Fougères système PPG les angiospermes avec leurs numéros de classement Lycophytes phylogénie phylogénétique. Herbier Introduction These alphabetical lists have been established for the systems of A.-L de Jussieu, A.-P. de Can- The organization of herbarium collections con- dolle, Bentham & Hooker, etc. that are still used sists in arranging the specimens logically to in the management of historical herbaria find and reclassify them easily in the appro- whose original classification is voluntarily pre- priate storage units. In the vascular plant col- served. lections, commonly used methods are systema- Recent classification systems based on molecu- tic classification, alphabetical classification, or lar phylogenies have developed, and herbaria combinations of both. -

Introduction to Common Native & Invasive Freshwater Plants in Alaska
Introduction to Common Native & Potential Invasive Freshwater Plants in Alaska Cover photographs by (top to bottom, left to right): Tara Chestnut/Hannah E. Anderson, Jamie Fenneman, Vanessa Morgan, Dana Visalli, Jamie Fenneman, Lynda K. Moore and Denny Lassuy. Introduction to Common Native & Potential Invasive Freshwater Plants in Alaska This document is based on An Aquatic Plant Identification Manual for Washington’s Freshwater Plants, which was modified with permission from the Washington State Department of Ecology, by the Center for Lakes and Reservoirs at Portland State University for Alaska Department of Fish and Game US Fish & Wildlife Service - Coastal Program US Fish & Wildlife Service - Aquatic Invasive Species Program December 2009 TABLE OF CONTENTS TABLE OF CONTENTS Acknowledgments ............................................................................ x Introduction Overview ............................................................................. xvi How to Use This Manual .................................................... xvi Categories of Special Interest Imperiled, Rare and Uncommon Aquatic Species ..................... xx Indigenous Peoples Use of Aquatic Plants .............................. xxi Invasive Aquatic Plants Impacts ................................................................................. xxi Vectors ................................................................................. xxii Prevention Tips .................................................... xxii Early Detection and Reporting -

Complete Chloroplast Genomes Shed Light on Phylogenetic
www.nature.com/scientificreports OPEN Complete chloroplast genomes shed light on phylogenetic relationships, divergence time, and biogeography of Allioideae (Amaryllidaceae) Ju Namgung1,4, Hoang Dang Khoa Do1,2,4, Changkyun Kim1, Hyeok Jae Choi3 & Joo‑Hwan Kim1* Allioideae includes economically important bulb crops such as garlic, onion, leeks, and some ornamental plants in Amaryllidaceae. Here, we reported the complete chloroplast genome (cpDNA) sequences of 17 species of Allioideae, fve of Amaryllidoideae, and one of Agapanthoideae. These cpDNA sequences represent 80 protein‑coding, 30 tRNA, and four rRNA genes, and range from 151,808 to 159,998 bp in length. Loss and pseudogenization of multiple genes (i.e., rps2, infA, and rpl22) appear to have occurred multiple times during the evolution of Alloideae. Additionally, eight mutation hotspots, including rps15-ycf1, rps16-trnQ-UUG, petG-trnW-CCA , psbA upstream, rpl32- trnL-UAG , ycf1, rpl22, matK, and ndhF, were identifed in the studied Allium species. Additionally, we present the frst phylogenomic analysis among the four tribes of Allioideae based on 74 cpDNA coding regions of 21 species of Allioideae, fve species of Amaryllidoideae, one species of Agapanthoideae, and fve species representing selected members of Asparagales. Our molecular phylogenomic results strongly support the monophyly of Allioideae, which is sister to Amaryllioideae. Within Allioideae, Tulbaghieae was sister to Gilliesieae‑Leucocoryneae whereas Allieae was sister to the clade of Tulbaghieae‑ Gilliesieae‑Leucocoryneae. Molecular dating analyses revealed the crown age of Allioideae in the Eocene (40.1 mya) followed by diferentiation of Allieae in the early Miocene (21.3 mya). The split of Gilliesieae from Leucocoryneae was estimated at 16.5 mya. -

Gondwanan Origin of Major Monocot Groups Inferred from Dispersal-Vicariance Analysis Kåre Bremer Uppsala University
Aliso: A Journal of Systematic and Evolutionary Botany Volume 22 | Issue 1 Article 3 2006 Gondwanan Origin of Major Monocot Groups Inferred from Dispersal-Vicariance Analysis Kåre Bremer Uppsala University Thomas Janssen Muséum National d'Histoire Naturelle Follow this and additional works at: http://scholarship.claremont.edu/aliso Part of the Botany Commons Recommended Citation Bremer, Kåre and Janssen, Thomas (2006) "Gondwanan Origin of Major Monocot Groups Inferred from Dispersal-Vicariance Analysis," Aliso: A Journal of Systematic and Evolutionary Botany: Vol. 22: Iss. 1, Article 3. Available at: http://scholarship.claremont.edu/aliso/vol22/iss1/3 Aliso 22, pp. 22-27 © 2006, Rancho Santa Ana Botanic Garden GONDWANAN ORIGIN OF MAJOR MONO COT GROUPS INFERRED FROM DISPERSAL-VICARIANCE ANALYSIS KARE BREMERl.3 AND THOMAS JANSSEN2 lDepartment of Systematic Botany, Evolutionary Biology Centre, Norbyvagen l8D, SE-752 36 Uppsala, Sweden; 2Museum National d'Histoire Naturelle, Departement de Systematique et Evolution, USM 0602: Taxonomie et collections, 16 rue Buffon, 75005 Paris, France 3Corresponding author ([email protected]) ABSTRACT Historical biogeography of major monocot groups was investigated by biogeographical analysis of a dated phylogeny including 79 of the 81 monocot families using the Angiosperm Phylogeny Group II (APG II) classification. Five major areas were used to describe the family distributions: Eurasia, North America, South America, Africa including Madagascar, and Australasia including New Guinea, New Caledonia, and New Zealand. In order to investigate the possible correspondence with continental breakup, the tree with its terminal distributions was fitted to the geological area cladogram «Eurasia, North America), (Africa, (South America, Australasia») and to alternative area cladograms using the TreeFitter program. -

Download the Full Report Pdf, 2.9 MB
VKM Report 2016:50 Assessment of the risks to Norwegian biodiversity from the import and keeping of aquarium and garden pond plants Opinion of the Panel on Alien Organisms and Trade in Endangered Species (CITES) of the Norwegian Scientific Committee for Food Safety Report from the Norwegian Scientific Committee for Food Safety (VKM) 2016:50 Assessment of the risks to Norwegian biodiversity from the import and keeping of aquarium and garden pond plants Opinion of the Panel on Alien Organisms and Trade in Endangered Species (CITES) of the Norwegian Scientific Committee for Food Safety 01.11.2016 ISBN: 00000-00000 Norwegian Scientific Committee for Food Safety (VKM) Po 4404 Nydalen N – 0403 Oslo Norway Phone: +47 21 62 28 00 Email: [email protected] www.vkm.no www.english.vkm.no Suggested citation: VKM (2016). Assessment of the risks to Norwegian biodiversity from the import and keeping of aquarium and garden pond plants. Scientific Opinion on the on Alien Organisms and Trade in Endangered species of the Norwegian Scientific Committee for Food Safety ISBN: 978-82-8259-240-6, Oslo, Norway. VKM Report 2016:50 Title: Assessment of the risks to Norwegian biodiversity from the import and keeping of aquarium and garden pond plants Authors preparing the draft opinion Hugo de Boer (chair), Maria G. Asmyhr (VKM staff), Hanne H. Grundt, Inga Kjersti Sjøtun, Hans K. Stenøien, Iris Stiers. Assessed and approved The opinion has been assessed and approved by Panel on Alien organisms and Trade in Endangered Species (CITES). Members of the panel are: Vigdis Vandvik (chair), Hugo de Boer, Jan Ove Gjershaug, Kjetil Hindar, Lawrence Kirkendall, Nina Elisabeth Nagy, Anders Nielsen, Eli K. -

Acorus Calamus : an Overview
Journal of Medicinal Plants Research Vol. 4(25), pp. 2740-2745, December Special Review, 2010 Available online at http://www.academicjournals.org/JMPR ISSN 1996-0875 ©2010 Academic Journals Review Acorus calamus : An overview R. Balakumbahan*, K. Rajamani and K. Kumanan Horticultural Research Station, TamilNadu Agricultural University, Pechiparai, 629161. Tamilnadu, India. Accepted 8 July, 2010 Acorus calamus (Sweet flag) is a wetland perennial monocot plant, in which the scented leaves and rhizomes have been traditionally used medicinally against different ailments like, fever, asthma, bronchitis, cough and mainly for digestive problems such as gas, bloating, colic, and poor digestive function. Number of active constituents and essential oil were identified and characterized from the leaves and rhizomes of sweet flag. An over view of the pharmacological activities and insecticidal activities are summarized here. Key words: Acorus calamus, Acorus gramineus , Acoraceae, active constituents, pharmacology. INTRODUCTION Mother earth has bestowed to the mankind and various Estimate reveals that the world trade in medicinal plants plants with healing ability for curing the ailments of and extracts industry was growing at a rate of 12 to 15% human being. This unique feature has been identified per annum. The export from India is to the tune of Rs 446 since pre historic times. The WHO has also estimated crores with the present growth rate of 7%. Acorus that 80% of the world population meets their primary calamus is a tall perennial wetland monocot plant from health care needs through traditional medicine only. the Acoraceae family. The scented leaves and rhizomes Medicinal plants are those plants possessing secondary of sweet flag have been traditionally used as a medicine metabolites and are potential sources of curative drugs and the dried and powdered rhizome has a spicy flavour with the very long list of chemicals and its curative nature. -
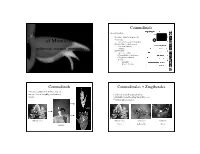
Diversity and Evolution of Monocots
Commelinids 4 main groups: Diversity and Evolution • Acorales - sister to all monocots • Alismatids of Monocots – inc. Aroids - jack in the pulpit • Lilioids (lilies, orchids, yams) – non-monophyletic . spiderworts, bananas, pineapples . – petaloid • Commelinids – Arecales – palms – Commelinales – spiderwort – Zingiberales –banana – Poales – pineapple – grasses & sedges Commelinids Commelinales + Zingiberales • theme: reduction of flower, loss of nectar, loss of zoophily, evolution of • 2 closely related tropical orders bracts • primarily nectar bearing but with losses • bracted inflorescences grass pickeral weed pickeral weed spiderwort heliconia nectar pollen only bracts rapatead bromeliad Commelinaceae - spiderwort Commelinaceae - spiderwort Family of small herbs with succulent stems, stems jointed; leaves sheathing. Family does not produce Inflorescence often bracted nectar, but showy flowers for insect pollen gathering. Rhoeo - Moses in a cradle Commelina erecta - Erect dayflower Tradescantia ohiensis - spiderwort Tradescantia ohiensis - spiderwort Commelinaceae - spiderwort Commelinaceae - spiderwort Flowers actinomorphic or • species rich in pantropics, CA 3 CO 3 A 6 G (3) zygomorphic especially Africa • floral diversity is enormous Commelina communis - day flower Tradescantia ohiensis - spiderwort Pontederiaceae - pickerel weed Pontederiaceae - pickerel weed Aquatic family of emergents or floaters. Pickerel weed has glossy heart-shaped leaves, Water hyacinth (Eichhornia) from superficially like Sagittaria but without net venation.