Supporting Information Appendix Pliocene Reversal of Late Neogene
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

December 8-10Am Fancy, and My Camera Thank Goodness Because the Subsequent Years Produced None Or Hardly Any Orchids
W: https://austplants.com.au/Southern-Tablelands E: [email protected] ACN 002 680 408 N E W S L E T T E R D E C E M B E R 2020 ROTARY MARKETS PLANT SALE dentata; occasional specimens of Dampiera stricta also provided blue colour. This was held a few weeks back and was our first plant sale in just over The tall sedge, Lepidosperma neesii was widespread and very 12 months. It also turned out to be our most lucrative sale with the profit healthy looking. We saw one burnt specimen of Melaleuca making its way into four figures. From the moment we had the ‘tent’ in hypericifolia which can display red ‘bottlebrush’ flowers; no flowers place we were besieged by buyers who seemed to know what they on this specimen - just some leaves and some seed pods to wanted. In the Riversdale sale last year, a number of buyers had been identify it. attracted by the display of the large ‘billy buttons - Pycnosorus The new growth did not make some plant identifications easier. globosus. While we had a few of those at the recent sale, they did not We are generally used to identifying species when they are mature attract much attention. It is likely of course that the success of the sale specimens. With re-growth, floral parts are often missing thus is at least partly related to the current situation in society; there is some leaving a vital clue out of the picture. belief that people want to get out and do things once again. -

Partial Flora Survey Rottnest Island Golf Course
PARTIAL FLORA SURVEY ROTTNEST ISLAND GOLF COURSE Prepared by Marion Timms Commencing 1 st Fairway travelling to 2 nd – 11 th left hand side Family Botanical Name Common Name Mimosaceae Acacia rostellifera Summer scented wattle Dasypogonaceae Acanthocarpus preissii Prickle lily Apocynaceae Alyxia Buxifolia Dysentry bush Casuarinacea Casuarina obesa Swamp sheoak Cupressaceae Callitris preissii Rottnest Is. Pine Chenopodiaceae Halosarcia indica supsp. Bidens Chenopodiaceae Sarcocornia blackiana Samphire Chenopodiaceae Threlkeldia diffusa Coast bonefruit Chenopodiaceae Sarcocornia quinqueflora Beaded samphire Chenopodiaceae Suada australis Seablite Chenopodiaceae Atriplex isatidea Coast saltbush Poaceae Sporabolis virginicus Marine couch Myrtaceae Melaleuca lanceolata Rottnest Is. Teatree Pittosporaceae Pittosporum phylliraeoides Weeping pittosporum Poaceae Stipa flavescens Tussock grass 2nd – 11 th Fairway Family Botanical Name Common Name Chenopodiaceae Sarcocornia quinqueflora Beaded samphire Chenopodiaceae Atriplex isatidea Coast saltbush Cyperaceae Gahnia trifida Coast sword sedge Pittosporaceae Pittosporum phyliraeoides Weeping pittosporum Myrtaceae Melaleuca lanceolata Rottnest Is. Teatree Chenopodiaceae Sarcocornia blackiana Samphire Central drainage wetland commencing at Vietnam sign Family Botanical Name Common Name Chenopodiaceae Halosarcia halecnomoides Chenopodiaceae Sarcocornia quinqueflora Beaded samphire Chenopodiaceae Sarcocornia blackiana Samphire Poaceae Sporobolis virginicus Cyperaceae Gahnia Trifida Coast sword sedge -

Cunninghamia Date of Publication: February 2020 a Journal of Plant Ecology for Eastern Australia
Cunninghamia Date of Publication: February 2020 A journal of plant ecology for eastern Australia ISSN 0727- 9620 (print) • ISSN 2200 - 405X (Online) The Australian paintings of Marianne North, 1880–1881: landscapes ‘doomed shortly to disappear’ John Leslie Dowe Australian Tropical Herbarium, James Cook University, Smithfield, Qld 4878 AUSTRALIA. [email protected] Abstract: The 80 paintings of Australian flora, fauna and landscapes by English artist Marianne North (1830-1890), completed during her travels in 1880–1881, provide a record of the Australian environment rarely presented by artists at that time. In the words of her mentor Sir Joseph Dalton Hooker, director of Kew Gardens, North’s objective was to capture landscapes that were ‘doomed shortly to disappear before the axe and the forest fires, the plough and the flock, or the ever advancing settler or colonist’. In addition to her paintings, North wrote books recollecting her travels, in which she presented her observations and explained the relevance of her paintings, within the principles of a ‘Darwinian vision,’ and inevitable and rapid environmental change. By examining her paintings and writings together, North’s works provide a documented narrative of the state of the Australian environment in the late nineteenth- century, filtered through the themes of personal botanical discovery, colonial expansion and British imperialism. Cunninghamia (2020) 20: 001–033 doi: 10.7751/cunninghamia.2020.20.001 Cunninghamia: a journal of plant ecology for eastern Australia © 2020 Royal Botanic Gardens and Domain Trust www.rbgsyd.nsw.gov.au/science/Scientific_publications/cunninghamia 2 Cunninghamia 20: 2020 John Dowe, Australian paintings of Marianne North, 1880–1881 Introduction The Marianne North Gallery in the Royal Botanic Gardens Kew houses 832 oil paintings which Marianne North (b. -

Alphabetical Lists of the Vascular Plant Families with Their Phylogenetic
Colligo 2 (1) : 3-10 BOTANIQUE Alphabetical lists of the vascular plant families with their phylogenetic classification numbers Listes alphabétiques des familles de plantes vasculaires avec leurs numéros de classement phylogénétique FRÉDÉRIC DANET* *Mairie de Lyon, Espaces verts, Jardin botanique, Herbier, 69205 Lyon cedex 01, France - [email protected] Citation : Danet F., 2019. Alphabetical lists of the vascular plant families with their phylogenetic classification numbers. Colligo, 2(1) : 3- 10. https://perma.cc/2WFD-A2A7 KEY-WORDS Angiosperms family arrangement Summary: This paper provides, for herbarium cura- Gymnosperms Classification tors, the alphabetical lists of the recognized families Pteridophytes APG system in pteridophytes, gymnosperms and angiosperms Ferns PPG system with their phylogenetic classification numbers. Lycophytes phylogeny Herbarium MOTS-CLÉS Angiospermes rangement des familles Résumé : Cet article produit, pour les conservateurs Gymnospermes Classification d’herbier, les listes alphabétiques des familles recon- Ptéridophytes système APG nues pour les ptéridophytes, les gymnospermes et Fougères système PPG les angiospermes avec leurs numéros de classement Lycophytes phylogénie phylogénétique. Herbier Introduction These alphabetical lists have been established for the systems of A.-L de Jussieu, A.-P. de Can- The organization of herbarium collections con- dolle, Bentham & Hooker, etc. that are still used sists in arranging the specimens logically to in the management of historical herbaria find and reclassify them easily in the appro- whose original classification is voluntarily pre- priate storage units. In the vascular plant col- served. lections, commonly used methods are systema- Recent classification systems based on molecu- tic classification, alphabetical classification, or lar phylogenies have developed, and herbaria combinations of both. -
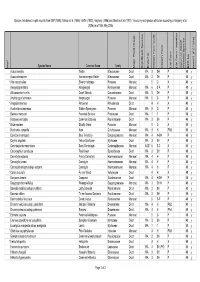
BFS048 Site Species List
Species lists based on plot records from DEP (1996), Gibson et al. (1994), Griffin (1993), Keighery (1996) and Weston et al. (1992). Taxonomy and species attributes according to Keighery et al. (2006) as of 16th May 2005. Species Name Common Name Family Major Plant Group Significant Species Endemic Growth Form Code Growth Form Life Form Life Form - aquatics Common SSCP Wetland Species BFS No kens01 (FCT23a) Wd? Acacia sessilis Wattle Mimosaceae Dicot WA 3 SH P 48 y Acacia stenoptera Narrow-winged Wattle Mimosaceae Dicot WA 3 SH P 48 y * Aira caryophyllea Silvery Hairgrass Poaceae Monocot 5 G A 48 y Alexgeorgea nitens Alexgeorgea Restionaceae Monocot WA 6 S-R P 48 y Allocasuarina humilis Dwarf Sheoak Casuarinaceae Dicot WA 3 SH P 48 y Amphipogon turbinatus Amphipogon Poaceae Monocot WA 5 G P 48 y * Anagallis arvensis Pimpernel Primulaceae Dicot 4 H A 48 y Austrostipa compressa Golden Speargrass Poaceae Monocot WA 5 G P 48 y Banksia menziesii Firewood Banksia Proteaceae Dicot WA 1 T P 48 y Bossiaea eriocarpa Common Bossiaea Papilionaceae Dicot WA 3 SH P 48 y * Briza maxima Blowfly Grass Poaceae Monocot 5 G A 48 y Burchardia congesta Kara Colchicaceae Monocot WA 4 H PAB 48 y Calectasia narragara Blue Tinsel Lily Dasypogonaceae Monocot WA 4 H-SH P 48 y Calytrix angulata Yellow Starflower Myrtaceae Dicot WA 3 SH P 48 y Centrolepis drummondiana Sand Centrolepis Centrolepidaceae Monocot AUST 6 S-C A 48 y Conostephium pendulum Pearlflower Epacridaceae Dicot WA 3 SH P 48 y Conostylis aculeata Prickly Conostylis Haemodoraceae Monocot WA 4 H P 48 y Conostylis juncea Conostylis Haemodoraceae Monocot WA 4 H P 48 y Conostylis setigera subsp. -

Gondwanan Origin of Major Monocot Groups Inferred from Dispersal-Vicariance Analysis Kåre Bremer Uppsala University
Aliso: A Journal of Systematic and Evolutionary Botany Volume 22 | Issue 1 Article 3 2006 Gondwanan Origin of Major Monocot Groups Inferred from Dispersal-Vicariance Analysis Kåre Bremer Uppsala University Thomas Janssen Muséum National d'Histoire Naturelle Follow this and additional works at: http://scholarship.claremont.edu/aliso Part of the Botany Commons Recommended Citation Bremer, Kåre and Janssen, Thomas (2006) "Gondwanan Origin of Major Monocot Groups Inferred from Dispersal-Vicariance Analysis," Aliso: A Journal of Systematic and Evolutionary Botany: Vol. 22: Iss. 1, Article 3. Available at: http://scholarship.claremont.edu/aliso/vol22/iss1/3 Aliso 22, pp. 22-27 © 2006, Rancho Santa Ana Botanic Garden GONDWANAN ORIGIN OF MAJOR MONO COT GROUPS INFERRED FROM DISPERSAL-VICARIANCE ANALYSIS KARE BREMERl.3 AND THOMAS JANSSEN2 lDepartment of Systematic Botany, Evolutionary Biology Centre, Norbyvagen l8D, SE-752 36 Uppsala, Sweden; 2Museum National d'Histoire Naturelle, Departement de Systematique et Evolution, USM 0602: Taxonomie et collections, 16 rue Buffon, 75005 Paris, France 3Corresponding author ([email protected]) ABSTRACT Historical biogeography of major monocot groups was investigated by biogeographical analysis of a dated phylogeny including 79 of the 81 monocot families using the Angiosperm Phylogeny Group II (APG II) classification. Five major areas were used to describe the family distributions: Eurasia, North America, South America, Africa including Madagascar, and Australasia including New Guinea, New Caledonia, and New Zealand. In order to investigate the possible correspondence with continental breakup, the tree with its terminal distributions was fitted to the geological area cladogram «Eurasia, North America), (Africa, (South America, Australasia») and to alternative area cladograms using the TreeFitter program. -

Introduction Methods Results
Papers and Proceedings Royal Society ofTasmania, Volume 1999 103 THE CHARACTERISTICS AND MANAGEMENT PROBLEMS OF THE VEGETATION AND FLORA OF THE HUNTINGFIELD AREA, SOUTHERN TASMANIA by J.B. Kirkpatrick (with two tables, four text-figures and one appendix) KIRKPATRICK, J.B., 1999 (31:x): The characteristics and management problems of the vegetation and flora of the Huntingfield area, southern Tasmania. Pap. Proc. R. Soc. Tasm. 133(1): 103-113. ISSN 0080-4703. School of Geography and Environmental Studies, University ofTasmania, GPO Box 252-78, Hobart, Tasmania, Australia 7001. The Huntingfield area has a varied vegetation, including substantial areas ofEucalyptus amygdalina heathy woodland, heath, buttongrass moorland and E. amygdalina shrubbyforest, with smaller areas ofwetland, grassland and E. ovata shrubbyforest. Six floristic communities are described for the area. Two hundred and one native vascular plant taxa, 26 moss species and ten liverworts are known from the area, which is particularly rich in orchids, two ofwhich are rare in Tasmania. Four other plant species are known to be rare and/or unreserved inTasmania. Sixty-four exotic plantspecies have been observed in the area, most ofwhich do not threaten the native biodiversity. However, a group offire-adapted shrubs are potentially serious invaders. Management problems in the area include the maintenance ofopen areas, weed invasion, pathogen invasion, introduced animals, fire, mechanised recreation, drainage from houses and roads, rubbish dumping and the gathering offirewood, sand and plants. Key Words: flora, forest, heath, Huntingfield, management, Tasmania, vegetation, wetland, woodland. INTRODUCTION species with the most cover in the shrub stratum (dominant species) was noted. If another species had more than half The Huntingfield Estate, approximately 400 ha of forest, the cover ofthe dominant one it was noted as a codominant. -

GENOME EVOLUTION in MONOCOTS a Dissertation
GENOME EVOLUTION IN MONOCOTS A Dissertation Presented to The Faculty of the Graduate School At the University of Missouri In Partial Fulfillment Of the Requirements for the Degree Doctor of Philosophy By Kate L. Hertweck Dr. J. Chris Pires, Dissertation Advisor JULY 2011 The undersigned, appointed by the dean of the Graduate School, have examined the dissertation entitled GENOME EVOLUTION IN MONOCOTS Presented by Kate L. Hertweck A candidate for the degree of Doctor of Philosophy And hereby certify that, in their opinion, it is worthy of acceptance. Dr. J. Chris Pires Dr. Lori Eggert Dr. Candace Galen Dr. Rose‐Marie Muzika ACKNOWLEDGEMENTS I am indebted to many people for their assistance during the course of my graduate education. I would not have derived such a keen understanding of the learning process without the tutelage of Dr. Sandi Abell. Members of the Pires lab provided prolific support in improving lab techniques, computational analysis, greenhouse maintenance, and writing support. Team Monocot, including Dr. Mike Kinney, Dr. Roxi Steele, and Erica Wheeler were particularly helpful, but other lab members working on Brassicaceae (Dr. Zhiyong Xiong, Dr. Maqsood Rehman, Pat Edger, Tatiana Arias, Dustin Mayfield) all provided vital support as well. I am also grateful for the support of a high school student, Cady Anderson, and an undergraduate, Tori Docktor, for their assistance in laboratory procedures. Many people, scientist and otherwise, helped with field collections: Dr. Travis Columbus, Hester Bell, Doug and Judy McGoon, Julie Ketner, Katy Klymus, and William Alexander. Many thanks to Barb Sonderman for taking care of my greenhouse collection of many odd plants brought back from the field. -

The Chloroplast Genome of Arthropodium Bifurcatum
Copyright is owned by the Author of the thesis. Permission is given for a copy to be downloaded by an individual for the purpose of research and private study only. The thesis may not be reproduced elsewhere without the permission of the Author. The Chloroplast Genome of Arthropodium bifurcatum. A thesis presented in partial fulfillment of the requirements for the degree of Master of Science in Biological Sciences Massey University Palmerston North, New Zealand Simon James Lethbridge Cox 2010 Abstract This thesis describes the application of high throughput (Illumina) short read sequencing and analyses to obtain the chloroplast genome sequence of Arthropodium bifurcatum and chloroplast genome markers for future testing of hypotheses that explain geographic distributions of Rengarenga – the name Maori give to species of Arthropodium in New Zealand. It has been proposed that A.cirratum was translocated from regions in the north of New Zealand to zones further south due to its value as a food crop. In order to develop markers to test this hypothesis, the chloroplast genome of the closely related A.bifurcatum was sequenced and annotated. A range of tools were used to handle the large quantities of data produced by the Illumina GAIIx. Programs included the de novo assembler Velvet, alignment tools BWA and Bowtie, the viewer Tablet and the quality control program SolexaQA. The A.bifurcatum genome was then used as a reference to align long range PCR products amplified from multiple accessions of A.cirratum and A.bifurcatum sampled from a range of geographic locations. From this alignment variable SNP markers were identified. -

TELOPEA Publication Date: 13 October 1983 Til
Volume 2(4): 425–452 TELOPEA Publication Date: 13 October 1983 Til. Ro)'al BOTANIC GARDENS dx.doi.org/10.7751/telopea19834408 Journal of Plant Systematics 6 DOPII(liPi Tmst plantnet.rbgsyd.nsw.gov.au/Telopea • escholarship.usyd.edu.au/journals/index.php/TEL· ISSN 0312-9764 (Print) • ISSN 2200-4025 (Online) Telopea 2(4): 425-452, Fig. 1 (1983) 425 CURRENT ANATOMICAL RESEARCH IN LILIACEAE, AMARYLLIDACEAE AND IRIDACEAE* D.F. CUTLER AND MARY GREGORY (Accepted for publication 20.9.1982) ABSTRACT Cutler, D.F. and Gregory, Mary (Jodrell(Jodrel/ Laboratory, Royal Botanic Gardens, Kew, Richmond, Surrey, England) 1983. Current anatomical research in Liliaceae, Amaryllidaceae and Iridaceae. Telopea 2(4): 425-452, Fig.1-An annotated bibliography is presented covering literature over the period 1968 to date. Recent research is described and areas of future work are discussed. INTRODUCTION In this article, the literature for the past twelve or so years is recorded on the anatomy of Liliaceae, AmarylIidaceae and Iridaceae and the smaller, related families, Alliaceae, Haemodoraceae, Hypoxidaceae, Ruscaceae, Smilacaceae and Trilliaceae. Subjects covered range from embryology, vegetative and floral anatomy to seed anatomy. A format is used in which references are arranged alphabetically, numbered and annotated, so that the reader can rapidly obtain an idea of the range and contents of papers on subjects of particular interest to him. The main research trends have been identified, classified, and check lists compiled for the major headings. Current systematic anatomy on the 'Anatomy of the Monocotyledons' series is reported. Comment is made on areas of research which might prove to be of future significance. -

Listado De Todas Las Plantas Que Tengo Fotografiadas Ordenado Por Familias Según El Sistema APG III (Última Actualización: 2 De Septiembre De 2021)
Listado de todas las plantas que tengo fotografiadas ordenado por familias según el sistema APG III (última actualización: 2 de Septiembre de 2021) GÉNERO Y ESPECIE FAMILIA SUBFAMILIA GÉNERO Y ESPECIE FAMILIA SUBFAMILIA Acanthus hungaricus Acanthaceae Acanthoideae Metarungia longistrobus Acanthaceae Acanthoideae Acanthus mollis Acanthaceae Acanthoideae Odontonema callistachyum Acanthaceae Acanthoideae Acanthus spinosus Acanthaceae Acanthoideae Odontonema cuspidatum Acanthaceae Acanthoideae Aphelandra flava Acanthaceae Acanthoideae Odontonema tubaeforme Acanthaceae Acanthoideae Aphelandra sinclairiana Acanthaceae Acanthoideae Pachystachys lutea Acanthaceae Acanthoideae Aphelandra squarrosa Acanthaceae Acanthoideae Pachystachys spicata Acanthaceae Acanthoideae Asystasia gangetica Acanthaceae Acanthoideae Peristrophe speciosa Acanthaceae Acanthoideae Barleria cristata Acanthaceae Acanthoideae Phaulopsis pulchella Acanthaceae Acanthoideae Barleria obtusa Acanthaceae Acanthoideae Pseuderanthemum carruthersii ‘Rubrum’ Acanthaceae Acanthoideae Barleria repens Acanthaceae Acanthoideae Pseuderanthemum carruthersii var. atropurpureum Acanthaceae Acanthoideae Brillantaisia lamium Acanthaceae Acanthoideae Pseuderanthemum carruthersii var. reticulatum Acanthaceae Acanthoideae Brillantaisia owariensis Acanthaceae Acanthoideae Pseuderanthemum laxiflorum Acanthaceae Acanthoideae Brillantaisia ulugurica Acanthaceae Acanthoideae Pseuderanthemum laxiflorum ‘Purple Dazzler’ Acanthaceae Acanthoideae Crossandra infundibuliformis Acanthaceae Acanthoideae Ruellia -

A Review of Natural Values Within the 2013 Extension to the Tasmanian Wilderness World Heritage Area
A review of natural values within the 2013 extension to the Tasmanian Wilderness World Heritage Area Nature Conservation Report 2017/6 Department of Primary Industries, Parks, Water and Environment Hobart A review of natural values within the 2013 extension to the Tasmanian Wilderness World Heritage Area Jayne Balmer, Jason Bradbury, Karen Richards, Tim Rudman, Micah Visoiu, Shannon Troy and Naomi Lawrence. Department of Primary Industries, Parks, Water and Environment Nature Conservation Report 2017/6, September 2017 This report was prepared under the direction of the Department of Primary Industries, Parks, Water and Environment (World Heritage Program). Australian Government funds were contributed to the project through the World Heritage Area program. The views and opinions expressed in this report are those of the authors and do not necessarily reflect those of the Tasmanian or Australian Governments. ISSN 1441-0680 Copyright 2017 Crown in right of State of Tasmania Apart from fair dealing for the purposes of private study, research, criticism or review, as permitted under the Copyright act, no part may be reproduced by any means without permission from the Department of Primary Industries, Parks, Water and Environment. Published by Natural Values Conservation Branch Department of Primary Industries, Parks, Water and Environment GPO Box 44 Hobart, Tasmania, 7001 Front Cover Photograph of Eucalyptus regnans tall forest in the Styx Valley: Rob Blakers Cite as: Balmer, J., Bradbury, J., Richards, K., Rudman, T., Visoiu, M., Troy, S. and Lawrence, N. 2017. A review of natural values within the 2013 extension to the Tasmanian Wilderness World Heritage Area. Nature Conservation Report 2017/6, Department of Primary Industries, Parks, Water and Environment, Hobart.