3-6 May Issue-2017.Indd
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Literature Compass Editing Humphry Davy's
1 ‘Work in Progress in Romanticism’ Literature Compass Editing Humphry Davy’s Letters Tim Fulford, Andrew Lacey, Sharon Ruston An editorial team of Tim Fulford (De Montfort University) and Sharon Ruston (Lancaster University) (co-editors), and Jan Golinski (University of New Hampshire), Frank James (the Royal Institution of Great Britain), and David Knight1 (Durham University) (advisory editors) are currently preparing The Collected Letters of Sir Humphry Davy: a four-volume edition of the c. 1200 surviving letters of Davy (1778-1829) and his immediate circle, for publication with Oxford University Press, in both print and electronic forms, in 2020. Davy was one of the most significant and famous figures in the scientific and literary culture of early nineteenth-century Britain, Europe, and America. Davy’s scientific accomplishments were varied and numerous, including conducting pioneering research into the physiological effects of nitrous oxide (laughing gas); isolating potassium, calcium, and several other metals; inventing a miners’ safety lamp (the bicentenary of which was celebrated in 2015); developing the electrochemical protection of the copper sheeting of Royal Navy vessels; conserving the Herculaneum papyri; writing an influential text on agricultural chemistry; and seeking to improve the quality of optical glass. But Davy’s endeavours were not merely limited to science: he was also a poet, and moved in the same literary circles as Lord Byron, Samuel Taylor Coleridge, Robert Southey, and William Wordsworth. Since his death, Davy has rarely been out of the public mind. He is still the frequent subject of biographies (by, 1 David Knight died in 2018. David gave generously to the Davy Letters Project, and a two-day conference at Durham University was recently held in his memory. -

The Study of Hems Based on the Mechanically Activated Intermetallic Al12mg17 Powder
molecules Article The Study of HEMs Based on the Mechanically Activated Intermetallic Al12Mg17 Powder Sergei Sokolov * , Alexander Vorozhtsov, Vladimir Arkhipov and Ilya Zhukov Laboratory of Metallurgy Nanotechnologies, National Research Tomsk State University, Lenin Avenue, 36, 634050 Tomsk, Russia; [email protected] (A.V.); [email protected] (V.A.); [email protected] (I.Z.) * Correspondence: [email protected]; Tel.: +7-923-406-77-01 Academic Editor: Svatopluk Zeman Received: 30 May 2020; Accepted: 19 July 2020; Published: 5 August 2020 Abstract: In this work, Al–Mg intermetallic powders were characterized and obtained by melting, casting into a steel chill and subsequent mechanical activation in a planetary mill. The method for producing Al12Mg17 intermetallic powder is presented. The dispersity, morphology, chemical composition, and phase composition of the obtained powder materials were investigated. Certain thermodynamic properties of high-energy materials containing the Al-Mg powder after mechanical activation of various durations were investigated. The addition of the Al-Mg powders to the high-energy composition (synthetic rubber SKDM-80 + ammonium perchlorate AP + boron B) can significantly increase the burning rate by approximately 47% and the combustion heat by approximately 23% compared with the high-energy compositions without the Al-Mg powder. The addition of the Al12Mg17 powder obtained after 6 h of mechanical activation provides an increase in the burning rate by 8% (2.5 0.1 mm/s for the mechanically activated Al Mg powder and ± 12 17 2.3 0.1 mm/s for the commercially available powder) and an increase in the combustion heat by 3% ± (7.4 0.2 MJ/kg for the mechanically activated Al-Mg powder and 7.1 0.2 MJ/kg for the commercially ± ± available powder). -

Atomic History Project Background: If You Were Asked to Draw the Structure of an Atom, What Would You Draw?
Atomic History Project Background: If you were asked to draw the structure of an atom, what would you draw? Throughout history, scientists have accepted five major different atomic models. Our perception of the atom has changed from the early Greek model because of clues or evidence that have been gathered through scientific experiments. As more evidence was gathered, old models were discarded or improved upon. Your task is to trace the atomic theory through history. Task: 1. You will create a timeline of the history of the atomic model that includes all of the following components: A. Names of 15 of the 21 scientists listed below B. The year of each scientist’s discovery that relates to the structure of the atom C. 1- 2 sentences describing the importance of the discovery that relates to the structure of the atom Scientists for the timeline: *required to be included • Empedocles • John Dalton* • Ernest Schrodinger • Democritus* • J.J. Thomson* • Marie & Pierre Curie • Aristotle • Robert Millikan • James Chadwick* • Evangelista Torricelli • Ernest • Henri Becquerel • Daniel Bernoulli Rutherford* • Albert Einstein • Joseph Priestly • Niels Bohr* • Max Planck • Antoine Lavoisier* • Louis • Michael Faraday • Joseph Louis Proust DeBroglie* Checklist for the timeline: • Timeline is in chronological order (earliest date to most recent date) • Equal space is devoted to each year (as on a number line) • The eight (8) *starred scientists are included with correct dates of their discoveries • An additional seven (7) scientists of your choice (from -

John Dalton By: Period 8 Early Years and Education
John Dalton By: Period 8 Early Years and Education • John Dalton was born in the small British village of Eaglesfield, Cumberland, England to a Quaker family. • As a child, John did not have much formal education because his family was rather poor; however, he did acquire a basic foundation in reading, writing, and arithmetic at a nearby Quaker school. • A teacher by the name of John Fletcher took young John Dalton under his wing and introduced him to a great mentor, Elihu Robinson, who was a rich Quaker. • Elihu then agreed to tutor John in mathematics, science, and meteorology. Shortly after he ended his tutoring sessions with Elihu, Dalton began keeping a daily log of the weather and other matters of meteorology. Education continued • His studies of these weather conditions led him to develop theories and hypotheses about mixed gases and water vapor. • He kept this journal of weather recordings his entire life, which later aided him in his observations and recordings of atoms and elements. Accomplishments • In 1794, Dalton became the first • Dalton joined the Manchester to explain color blindness, Literary and Philosophical which he was afflicted with Society and instantly published himself, at one of his public his first book on Meteorological lectures and it is even Observations and Essays. sometimes called Daltonism referring to John Dalton • In this book, John tells of his himself. ideas on gasses and that “in a • The first paper he wrote on this mixture of gasses, each gas matter was entitled exists independently of each Extraordinary facts relating to other gas and acts accordingly,” the visions of colors “in which which was when he’s famous he postulated that shortage in ideas on the Atomic Theory color perception was caused by started to form. -
![Arxiv:0801.0028V1 [Physics.Atom-Ph] 29 Dec 2007 § ‡ † (People’S China Metrology, Of) of Lic Institute Canada National Council, Zhang, Research Z](https://docslib.b-cdn.net/cover/1910/arxiv-0801-0028v1-physics-atom-ph-29-dec-2007-%C2%A7-people-s-china-metrology-of-of-lic-institute-canada-national-council-zhang-research-z-651910.webp)
Arxiv:0801.0028V1 [Physics.Atom-Ph] 29 Dec 2007 § ‡ † (People’S China Metrology, Of) of Lic Institute Canada National Council, Zhang, Research Z
CODATA Recommended Values of the Fundamental Physical Constants: 2006∗ Peter J. Mohr†, Barry N. Taylor‡, and David B. Newell§, National Institute of Standards and Technology, Gaithersburg, Maryland 20899-8420, USA (Dated: March 29, 2012) This paper gives the 2006 self-consistent set of values of the basic constants and conversion factors of physics and chemistry recommended by the Committee on Data for Science and Technology (CODATA) for international use. Further, it describes in detail the adjustment of the values of the constants, including the selection of the final set of input data based on the results of least-squares analyses. The 2006 adjustment takes into account the data considered in the 2002 adjustment as well as the data that became available between 31 December 2002, the closing date of that adjustment, and 31 December 2006, the closing date of the new adjustment. The new data have led to a significant reduction in the uncertainties of many recommended values. The 2006 set replaces the previously recommended 2002 CODATA set and may also be found on the World Wide Web at physics.nist.gov/constants. Contents 3. Cyclotron resonance measurement of the electron relative atomic mass Ar(e) 8 Glossary 2 4. Atomic transition frequencies 8 1. Introduction 4 1. Hydrogen and deuterium transition frequencies, the 1. Background 4 Rydberg constant R∞, and the proton and deuteron charge radii R , R 8 2. Time variation of the constants 5 p d 1. Theory relevant to the Rydberg constant 9 3. Outline of paper 5 2. Experiments on hydrogen and deuterium 16 3. -

Conventioral Standards?* JESSE W
156 IRE TRANSACTIONS ON INSTRUMENTATION December Present Status of Precise Information on the Universal Physical Constants. Has the Time Arrived for Their Adoption to Replace Our Present Arbitrary Conventioral Standards?* JESSE W. M. DuMONDt INTRODUCTION this subject that the experimentally measured data r HREE years ago Dr. E. R. Cohen and I prepared often do not give the desired unknowns directly but and published our latest (1955) least-squares ad- instead give functions of the unknowns. justment of all the most reliable data then avail- Seven different functions of these above four un- able bearing on the universal constants of physics and knowns have been measured by experimental methods chemistry. Since then new data and information have which we feel are sufficiently precise and reliable to been accumulating so that a year or two from now the qualify them as input data in a least-squares adjust- time may perhaps be propitious for us to prepare a new ment. These seven experimentally determined numeri- adjustment taking the newly-gained knowledge into cal values are not only functions of the unknowns,ae, account. At present it is too early to attempt such a e, N, and A, but also of the above-mentioned experi re-evaluation since many of the investigations and re- mentally determined auxiliary constants, of which five determinations now under way are still far from com- different kinds are listed in Table I. Another of these pleted. I shall be obliged, therefore, to content myself auxiliary constants I find it expedient to recall to your in this talk with a description of the sources of informa- attention at the very beginning to avoid any possibility tion upon which our 1955 evaluation was based, men- of confusion. -

Atomic Theories and Models
Atomic Theories and Models Answer these questions on your own. Early Ideas About Atoms: Go to http://www.infoplease.com/ipa/A0905226.html and read the section on “Greek Origins” in order to answer the following: 1. What were Leucippus and Democritus ideas regarding matter? 2. Describe what these philosophers thought the atom looked like? 3. How were the ideas of these two men received by Aristotle, and what was the result on the progress of atomic theory for the next couple thousand years? Alchemists: Go to http://dictionary.reference.com/browse/alchemy and/or http://www.scienceandyou.org/articles/ess_08.shtml to answer the following: 4. What was the ultimate goal of an alchemist? 5. What is the word used to describe changing something of little value into something of higher value? 6. Did any alchemist achieve this goal? John Dalton’s Atomic Theory: Go to http://www.rsc.org/chemsoc/timeline/pages/1803.html and answer the following: 7. When did Dalton form his atomic theory. 8. List the six ideas of Dalton’s theory: a. b. c. d. e. f. Mendeleev: Go to http://www.chemistry.co.nz/mendeleev.htm and/or http://www.aip.org/history/curie/periodic.htm to answer the following: 9. What was Mendeleev’s famous contribution to chemistry? 10. How did Mendeleev arrange his periodic table? 11. Why did Mendeleev leave blank spaces in his periodic table? J. J. Thomson: Go to http://www.universetoday.com/38326/plum-pudding-model/ and http://www.chemheritage.org/discover/online-resources/chemistry-in-history/themes/atomic-and- nuclear-structure/thomson.aspx and http://www.chem.uiuc.edu/clcwebsite/cathode.html and http://www-outreach.phy.cam.ac.uk/camphy/electron/electron_index.htm and http://www.iun.edu/~cpanhd/C101webnotes/modern-atomic-theory/rutherford-model.html to answer the following: 12. -

1 Classical Theory and Atomistics
1 1 Classical Theory and Atomistics Many research workers have pursued the friction law. Behind the fruitful achievements, we found enormous amounts of efforts by workers in every kind of research field. Friction research has crossed more than 500 years from its beginning to establish the law of friction, and the long story of the scientific historyoffrictionresearchisintroducedhere. 1.1 Law of Friction Coulomb’s friction law1 was established at the end of the eighteenth century [1]. Before that, from the end of the seventeenth century to the middle of the eigh- teenth century, the basis or groundwork for research had already been done by Guillaume Amontons2 [2]. The very first results in the science of friction were found in the notes and experimental sketches of Leonardo da Vinci.3 In his exper- imental notes in 1508 [3], da Vinci evaluated the effects of surface roughness on the friction force for stone and wood, and, for the first time, presented the concept of a coefficient of friction. Coulomb’s friction law is simple and sensible, and we can readily obtain it through modern experimentation. This law is easily verified with current exper- imental techniques, but during the Renaissance era in Italy, it was not easy to carry out experiments with sufficient accuracy to clearly demonstrate the uni- versality of the friction law. For that reason, 300 years of history passed after the beginning of the Italian Renaissance in the fifteenth century before the friction law was established as Coulomb’s law. The progress of industrialization in England between 1750 and 1850, which was later called the Industrial Revolution, brought about a major change in the production activities of human beings in Western society and later on a global scale. -

The Discovery of Thermodynamics
Philosophical Magazine ISSN: 1478-6435 (Print) 1478-6443 (Online) Journal homepage: https://www.tandfonline.com/loi/tphm20 The discovery of thermodynamics Peter Weinberger To cite this article: Peter Weinberger (2013) The discovery of thermodynamics, Philosophical Magazine, 93:20, 2576-2612, DOI: 10.1080/14786435.2013.784402 To link to this article: https://doi.org/10.1080/14786435.2013.784402 Published online: 09 Apr 2013. Submit your article to this journal Article views: 658 Citing articles: 2 View citing articles Full Terms & Conditions of access and use can be found at https://www.tandfonline.com/action/journalInformation?journalCode=tphm20 Philosophical Magazine, 2013 Vol. 93, No. 20, 2576–2612, http://dx.doi.org/10.1080/14786435.2013.784402 COMMENTARY The discovery of thermodynamics Peter Weinberger∗ Center for Computational Nanoscience, Seilerstätte 10/21, A1010 Vienna, Austria (Received 21 December 2012; final version received 6 March 2013) Based on the idea that a scientific journal is also an “agora” (Greek: market place) for the exchange of ideas and scientific concepts, the history of thermodynamics between 1800 and 1910 as documented in the Philosophical Magazine Archives is uncovered. Famous scientists such as Joule, Thomson (Lord Kelvin), Clau- sius, Maxwell or Boltzmann shared this forum. Not always in the most friendly manner. It is interesting to find out, how difficult it was to describe in a scientific (mathematical) language a phenomenon like “heat”, to see, how long it took to arrive at one of the fundamental principles in physics: entropy. Scientific progress started from the simple rule of Boyle and Mariotte dating from the late eighteenth century and arrived in the twentieth century with the concept of probabilities. -

Aluminium Alloys Chemical Composition Pdf
Aluminium alloys chemical composition pdf Continue Alloy in which aluminum is the predominant lye frame of aluminum welded aluminium alloy, manufactured in 1990. Aluminum alloys (or aluminium alloys; see spelling differences) are alloys in which aluminium (Al) is the predominant metal. Typical alloy elements are copper, magnesium, manganese, silicon, tin and zinc. There are two main classifications, namely casting alloys and forged alloys, both further subdivided into heat-treatable and heat-free categories. Approximately 85% of aluminium is used for forged products, e.g. laminated plates, foils and extrusions. Aluminum cast alloys produce cost-effective products due to their low melting point, although they generally have lower tensile strength than forged alloys. The most important cast aluminium alloy system is Al–Si, where high silicon levels (4.0–13%) contributes to giving good casting features. Aluminum alloys are widely used in engineering structures and components where a low weight or corrosion resistance is required. [1] Alloys composed mostly of aluminium have been very important in aerospace production since the introduction of metal leather aircraft. Aluminum-magnesium alloys are both lighter than other aluminium alloys and much less flammable than other alloys containing a very high percentage of magnesium. [2] Aluminum alloy surfaces will develop a white layer, protective of aluminum oxide, if not protected by proper anodization and/or dyeing procedures. In a wet environment, galvanic corrosion can occur when an aluminum alloy is placed in electrical contact with other metals with a more positive corrosion potential than aluminum, and an electrolyte is present that allows the exchange of ions. -
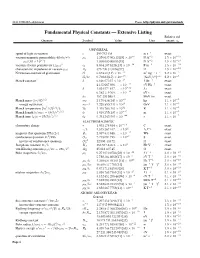
Fundamental Physical Constants — Extensive Listing Relative Std
2018 CODATA adjustment From: http://physics.nist.gov/constants Fundamental Physical Constants — Extensive Listing Relative std. Quantity Symbol Value Unit uncert. ur UNIVERSAL speed of light in vacuum c 299 792 458 m s−1 exact 2 −6 −2 −10 vacuum magnetic permeability 4pα¯h=e c µ0 1:256 637 062 12(19) × 10 NA 1:5 × 10 −7 −2 −10 µ0=(4p × 10 ) 1:000 000 000 55(15) NA 1:5 × 10 2 −12 −1 −10 vacuum electric permittivity 1/µ0c 0 8:854 187 8128(13) × 10 F m 1:5 × 10 −10 characteristic impedance of vacuum µ0c Z0 376:730 313 668(57) Ω 1:5 × 10 Newtonian constant of gravitation G 6:674 30(15) × 10−11 m3 kg−1 s−2 2:2 × 10−5 G=¯hc 6:708 83(15) × 10−39 (GeV=c2)−2 2:2 × 10−5 Planck constant∗ h 6:626 070 15 × 10−34 J Hz−1 exact 4:135 667 696 ::: × 10−15 eV Hz−1 exact ¯h 1:054 571 817 ::: × 10−34 J s exact 6:582 119 569 ::: × 10−16 eV s exact ¯hc 197:326 980 4 ::: MeV fm exact 1=2 −8 −5 Planck mass (¯hc=G) mP 2:176 434(24) × 10 kg 1:1 × 10 2 19 −5 energy equivalent mPc 1:220 890(14) × 10 GeV 1:1 × 10 5 1=2 32 −5 Planck temperature (¯hc =G) =k TP 1:416 784(16) × 10 K 1:1 × 10 3 1=2 −35 −5 Planck length ¯h=mPc = (¯hG=c ) lP 1:616 255(18) × 10 m 1:1 × 10 5 1=2 −44 −5 Planck time lP=c = (¯hG=c ) tP 5:391 247(60) × 10 s 1:1 × 10 ELECTROMAGNETIC elementary charge e 1:602 176 634 × 10−19 C exact e=¯h 1:519 267 447 ::: × 1015 AJ−1 exact −15 magnetic flux quantum 2p¯h=(2e) Φ0 2:067 833 848 ::: × 10 Wb exact 2 −5 conductance quantum 2e =2p¯h G0 7:748 091 729 ::: × 10 S exact −1 inverse of conductance quantum G0 12 906:403 72 ::: Ω exact 9 −1 Josephson constant 2e=h -

The World Around Is Physics
The world around is physics Life in science is hard What we see is engineering Chemistry is harder There is no money in chemistry Future is uncertain There is no need of chemistry Therefore, it is not my option I don’t have to learn chemistry Chemistry is life Chemistry is chemicals Chemistry is memorizing things Chemistry is smell Chemistry is this and that- not sure Chemistry is fumes Chemistry is boring Chemistry is pollution Chemistry does not excite Chemistry is poison Chemistry is a finished subject Chemistry is dirty Chemistry - stands on the legacy of giants Antoine-Laurent Lavoisier (1743-1794) Marie Skłodowska Curie (1867- 1934) John Dalton (1766- 1844) Sir Humphrey Davy (1778 – 1829) Michael Faraday (1791 – 1867) Chemistry – our legacy Mendeleev's Periodic Table Modern Periodic Table Dmitri Ivanovich Mendeleev (1834-1907) Joseph John Thomson (1856 –1940) Great experimentalists Ernest Rutherford (1871-1937) Jagadish Chandra Bose (1858 –1937) Chandrasekhara Venkata Raman (1888-1970) Chemistry and chemical bond Gilbert Newton Lewis (1875 –1946) Harold Clayton Urey (1893- 1981) Glenn Theodore Seaborg (1912- 1999) Linus Carl Pauling (1901– 1994) Master craftsmen Robert Burns Woodward (1917 – 1979) Chemistry and the world Fritz Haber (1868 – 1934) Machines in science R. E. Smalley Great teachers Graduate students : Other students : 1. Werner Heisenberg 1. Herbert Kroemer 2. Wolfgang Pauli 2. Linus Pauling 3. Peter Debye 3. Walter Heitler 4. Paul Sophus Epstein 4. Walter Romberg 5. Hans Bethe 6. Ernst Guillemin 7. Karl Bechert 8. Paul Peter Ewald 9. Herbert Fröhlich 10. Erwin Fues 11. Helmut Hönl 12. Ludwig Hopf 13. Walther Kossel 14.