Sperm-Egg Interaction During Fertilization in Birds
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Preliminary Note on the Embryology of <Emphasis Type="Italic">Casuarina Equisetifolia </Emphasis>, Forst
A PRELIMINARY NOTE ON THE EMBRYOLOGY OF CASUARINA EQUISETIFOLIA, FORST BY B. G. L. SWAMs (Bangalore) Received June 27, 1944 (Communicated by Prof. L. S. S. Kumar, r.A.SC.) THE remarkable discovery of Chalazogamy in Casuarina by Treub in 1891 evoked very keen interest and initiated further studies of Casuarinaceae and Amentifera~ fi'om both morphological and anatomical points of view. Certain aspects of the megasporogenesis of Casuarhza stricta was subsequently studied by Frye in 1903 and Juel (1903) recorded his observations on the origin and development of the female archesporium in Casuarina quadrivalvis. In spite of these contributions our present knowledge regarding the develop- mental stages in the life-history are far from being satisfactory. An inves- tigation of several species of the genus has been taken up by the author and a few salient features in the life-history of Casuarina equiset~folia Forst have been embodied in this preliminary note. The archesporiurn of the microsporangium is subepidermal in origin and can be differentiated by rich cell contents and conspicuous nuclei. After the formation of the endothecium, wall layers and tapetum, the microspore mother cells undergo the usual stages of the reduction divisions and form quartets of microspores arranged tetrahedrally. The quartets round off and their nuclei undergo division into tube and generative cells The pollen grains at the shedding stage are binucleate. Each ovary contains two erect ovules which arise laterally from a basal placenta (Fig. 1). The ovules are bitegnmentary, the inner integument differentiating slightly earlier than the outer; these grow upwards and organise a micropyle. -

Ostrich Production Systems Part I: a Review
11111111111,- 1SSN 0254-6019 Ostrich production systems Food and Agriculture Organization of 111160mmi the United Natiorp str. ro ucti s ct1rns Part A review by Dr M.M. ,,hanawany International Consultant Part II Case studies by Dr John Dingle FAO Visiting Scientist Food and , Agriculture Organization of the ' United , Nations Ot,i1 The designations employed and the presentation of material in this publication do not imply the expression of any opinion whatsoever on the part of the Food and Agriculture Organization of the United Nations concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. M-21 ISBN 92-5-104300-0 Reproduction of this publication for educational or other non-commercial purposes is authorized without any prior written permission from the copyright holders provided the source is fully acknowledged. Reproduction of this publication for resale or other commercial purposes is prohibited without written permission of the copyright holders. Applications for such permission, with a statement of the purpose and extent of the reproduction, should be addressed to the Director, Information Division, Food and Agriculture Organization of the United Nations, Viale dells Terme di Caracalla, 00100 Rome, Italy. C) FAO 1999 Contents PART I - PRODUCTION SYSTEMS INTRODUCTION Chapter 1 ORIGIN AND EVOLUTION OF THE OSTRICH 5 Classification of the ostrich in the animal kingdom 5 Geographical distribution of ratites 8 Ostrich subspecies 10 The North -

Protection of Washed and Pasteurized Shell Eggs Against Fungal Growth by Application of Natamycin-Containing Shellac Coating
Protection of Washed and Pasteurized Shell Eggs against Fungal Growth by Application of Natamycin-Containing Shellac Coating THESIS Presented in Partial Fulfillment of the Requirements for the Degree Master of Science in the Graduate School of The Ohio State University By Yang Song Graduate Program in Food Science and Technology The Ohio State University 2016 Master's Examination Committee: Dr. Ahmed Yousef, Advisor Dr. Dennis R. Heldman Dr. Luis Rodriguez-Saona Copyrighted by Yang Song 2016 Abstract Mold contamination of commercial shell eggs can potentially cause significant economic loss to the egg industry during storage. Studies indicated that molds from varies sources can propagate on commercial eggs when storage condition is less ideal. The current egg processing procedures such as commercial washing and pasteurization can weaken the egg shell, which is the primary defense of egg content, and expose processed eggs to contaminations. Generally, processed eggs are coated with mineral oil to overcome this problem. However, oil application is not very effective when used to protect eggs against mold contamination during storage. The food grade anti-fungal agent natamycin can be used to improve egg defense against mold contamination; however, direct application on egg surface will cause it to lose activity rapidly. Therefore, incorporation of natamycin and a food-grade coating is necessary to extend its anti-fungal effectiveness. As a food-grade coating, shellac can retain egg quality better compare to other coating materials; moreover, it can also serve as a matrix for natamycin to treat egg surface. Research is needed to investigate whether natamycin can remain effective in shellac coating; determine the minimum inhibitory concentration (MIC) of natamycin in shellac coating against typical mold contaminants, and whether the natamycin-shellac coating is effective when used on commercial washed eggs and pasteurized eggs. -

Mind Your Eggs & Veggies
MIND YOUR EGGS & VEGGIES Nutrition for Cognitive Health Presented by Taylor C. Wallace, PhD, CFS, FACN And Chef Abbie Gellman, MS, RD, CDN Spread the Fruit and Veggie Love #haveaplant @fruits_veggies @fruitsandveggies @fruitsandveggies © 2020 Produce for Better Health Foundation 2 Our Purpose The Produce for Better Health Foundation (PBH), a 501(c)3, is the only national non-profit organization committed to helping people live happier, healthier lives by eating more fruits and vegetables in all their glorious forms every day. © 20202019 Produce for Better Health Foundation 3 Our Movement Research shows, rather than a prescriptive recommendation to eat a certain amount of fruits and vegetables each day, consumers (particularly Gen Z and Millennials) want actionable, realistic and FUN approaches that make eating fruits and vegetables easy, helping them feel confident, happy and healthy. That’s where PBH’s Have A Plant® movement comes in. It’s a way to tap into the emotional connection consumers have to the fruit and vegetable eating experience while inspiring long-term, sustainable behavior change. And it does so with a no-nonsense approach that’s simple, understandable, and, importantly for this audience, non-prescriptive. © 2020 Produce for Better Health Foundation 4 Moderator Wendy Reinhardt Kapsak, MS, RDN © 2020 Produce for Better Health Foundation 5 Content Series: Eggs: Veggies’ Reliable BFF © 2020 Produce for Better Health Foundation 6 Adding Eggs to Enhance the Benefits of Produce Taylor C. Wallace, PhD, CFS, FACN Think Healthy Group, Inc. George Mason University © 2020 Produce for Better Health Foundation 7 Disclosures • Think Healthy Group, Inc. • George Mason University • The Dr. -

2020 Front Cover
INDIANA STATE POULTRY ASSOCIATION PRESENTS: 2020 POULTRY INFOMATION BOOKLET THIS BOOKLET CONTAINS: Test Your Knowledge - Poultry Quiz (Inside Front Cover) Poultry Terms Quick Reference Guide (Pg. 1) Getting Started with a Home Poultry Flock (Pg. 2) Common Egg Shell Quality Problems (Pg. 5) Choosing a Chicken Breed: Eggs, Meat or Exhibition (Pg. 6) Stay Healthy When Working with Farm Animals (Pg. 9) Exhibition Poultry (Pg. 10) Proper Handling of Eggs: From Hen to Consumption (Pg. 12) Don’t Get Caught Without These Forms (Pg. 17) Avian Influenza Findings Emphasize the Need for Good Biosecurity (Pg. 18) Backyard Biosecurity Self Evaluation (Pg. 20) Indiana Test Twelve Flock Evaluation (Inside Back Cover) This Booklet is designed to be a quick resource for raising poultry. For additional information go to www.INpoultry.com INDIANA STATE POULTRY ASSOCIATION PURDUE UNIVERSITY, ANIMAL SCIENCES 270 SOUTH RUSSELL STREET • WEST LAFAYETTE, IN 47907 765-494-8517 • [email protected] • WWW.INPOULTRY.COM TEST YOUR KNOWLEDGE - POULTRY QUIZ THINK YOU KNOW POULTRY? ANSWER THESE TEN QUESTIONS FOR A CHANCE TO WIN A PRIZE. ALL ANSWERS CAN BE FOUND THROUGHOUT THIS BOOKLET. 1. If chicks move away from the heat source in their brooding area and appear to be drowsy, then the temperature is most likely too ________ . 2. What might be the cause of a misshapen egg? a. Immature shell gland b. Disease c. Stress d. Overcrowding e. All of the above 3. Chicken breeds with _______ ear lobes lay white eggs while chicken breeds with ______ ear lobes lay brown eggs. 4. Anyone can get sick from farm animals, but what groups of people are more likely to have a serious illness? a. -

An Introduction to Morphology of the Reproductive System and Anatomy of Hen's
J. Life Earth Sci., Vol. 8: 1-10, 2013 ISSN 1990-4827 http://banglajol.info.index.php/JLES © 2013, JLES, RU AN INTRODUCTION TO MORPHOLOGY OF THE REPRODUCTIVE SYSTEM AND ANATOMY OF HEN’S EGG Md. Anisur Rahman Department of Zoology, University of Rajshahi, Rajshahi-6205, Bangladesh e-mail: [email protected] Abstract: The present study was designed to investigate the morphology of reproductive system and egg anatomy of the domestic hen (Gallus domesticus L.). The system consists of oviduct and ovary. The oviduct consists of infundibulum, magnum, isthmus, uterus and vagina which are sole distributor for making nutrition enriched egg. The anatomy of egg revealed that there are calcareous eggshell, shell membranes, egg white, vitelline membrane, egg yolk, and germinal disc. The fertilized egg showed a concentric circle around the nucleus known as blastoderm that contained area pellucida and area opaca whereas an unfertilized egg showed nucleus as white spot (blastodisc). Primordial germ cells (PGCs) are progenitor cells of ova and spermatozoa and they originate from the epiblast of the central part of the area pellucida. The microscopic structure of eggshell rendered leathery cuticle, fibrous matrix and shell membranes. The egg protects itself by its own mechanism from being injured and provides a complete diet for the developing embryo. Key words: Ovary, oviduct, egg, blastodisc, eggshell mvivsk: eZ©gvb Aa¨qbwU gyiwMi (Gallus domesticus L.) cÖRbbZ‡š¿i A½ms¯’vb Ges wW‡gi A½e¨e‡”Q` AbymÜvb Kivi Rb¨ Av‡jLb Kiv n‡q‡Q| cÖRbbZš¿wU‡Z wW¤^bvjx -
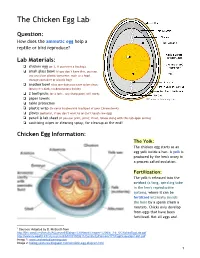
The Chicken Egg Lab 1
The Chicken Egg Lab1 Question: How does the amniotic egg help a reptile or bird reproduce? Lab Materials: ❏ chicken egg (or 2, if you want a backup) ❏ small glass bowl (if you don’t have this, you can use any clear plastic container, such as a food storage container or plastic bag) ❏ another bowl (this one does not have to be clear; ideally it’s dark, no decorations inside) ❏ 2 toothpicks (or a fork… any sharp point will work) ❏ paper towels ❏ table protection ❏ plastic wrap (to cover keyboard & trackpad of your Chromebook) ❏ gloves (optional, if you don’t want to or can’t touch raw egg) ❏ pencil & lab sheet (if you can print, print; if not, follow along with this tab open online) ❏ sanitizing wipes or cleaning spray, for cleanup at the end! Chicken Egg Information: The Yolk: The chicken egg starts as an egg yolk inside a hen. A yolk is produced by the hen's ovary in a process called ovulation. Fertilization: The yolk is released into the oviduct (a long, spiraling tube in the hen's reproductive system), where it can be fertilized internally (inside the hen) by a sperm (from a rooster). Chicks only develop from eggs that have been fertilized. Not all eggs are! 1 Sources: Adapted by D. McBeath from http://ljhs.sandi.net/faculty/AQuesnell/Biology%20Notes/Chapter%206/6_3-6_5/ChickenEggLab.pdf http://www.newpaltz.k12.ny.us/cms/lib/NY01000611/Centricity/Domain/171/EggDissectionLab1.pdf Image 1: www.enchantedlearning.com Image 2: biology-pictures.blogspot.com/amniotic-egg-diagram.html 1 The Egg White (albumin): The yolk continues down the oviduct (whether or not it is fertilized) and is covered with a membrane, structural fibers, and layers of albumin protein (the egg white). -

Review Yoshinori Mine and Jennifer Kovacs-Nolan
Journal of Poultry Science, .+ : +῎,3, ,**. ῌReview῍ Yoshinori Mine and Jennifer Kovacs-Nolan Department of Food Science, University of Guelph Guelph, Ontario N+G ,W+, Canada It is widely recognized that eggs are more than a source of dietary nutrients, and extensive studies identifying and characterizing the biologically active components of eggs have been carried out. Numerous biological activities have now been associated with egg components, including antibacterial and antiviral activity, immunomodulatory activity, and anti-cancer activity, indicating the importance of eggs and egg components in human health, and disease prevention and treatment. The potential of some of these biologically active components has already been realized, including egg white lysozyme and avidin, and yolk IgY and lecithin, which are currently produced on an industrial scale, and have been applied for the prevention and treatment of various medical conditions. The information presented here serves to demonstrate the significant potential of biologically active egg components, for medical, nutraceutical, and food- fortification applications. Key words : Hen eggs, bio-active components, nutraceuticals, health and disease, functional foods ῌῌῌῌῌῌῌῌῌῌῌῌῌῌῌῌῌῌῌῌῌῌῌ Yoshinori Mine +321 : M. Sc. (Food Science) Shinshu University, Japan +33- : Ph. D. (Biochemistry) Tokyo University of Agriculture and Technology, Japan Current position Associate Professor and Chair in Egg Material Sciences Department of Food Science University of Guelph. Area of research interests : Egg Material Sciences, Bio-active protein/peptides, Molecular biology of egg allergens. Jennifer Kovacs-Nolan ,**+ : M. Sc. (Food Science) University of Guelph , Ontario, Canada. Current position : Ph. D. student, Department of Food Science, University of Guelph, Ontario, Canada. Area of research interests : Food biochemistry/biotechnology. -

Novel Pickering High Internal Phase Emulsion Stabilized by Food Waste-Hen Egg Chalaza
foods Article Novel Pickering High Internal Phase Emulsion Stabilized by Food Waste-Hen Egg Chalaza Lijuan Wang 1, Jingjing Wang 1 and Anheng Wang 1,2,* 1 College of Grain Engineering and Technology, Shenyang Normal University, Shenyang 110034, China; [email protected] (L.W.); [email protected] (J.W.) 2 Department of Chemistry and Biochemistry, School of Mathematics and Physical Sciences (Chemistry), University of Hull, Cottingham Road, Hull HU67RX, UK * Correspondence: [email protected]; Tel.: +44-7421834574; Fax: +86-02486506860 Abstract: A massive amount of chalaza with nearly 400 metric tons is produced annually as waste in the liquid-egg industry. The present study aimed to look for ways to utilize chalaza as a natural emulsifier for high internal phase emulsions (HIPEs) at the optimal production conditions to expand the utilization of such abundant material. To the author’s knowledge, for the first time, we report the usage of hen egg chalaza particles as particulate emulsifiers for Pickering (HIPEs) development. The chalaza particles with partial wettability were fabricated at different pH or ionic strengths by freeze-drying. The surface electricity of the chalaza particles was neutralized when the pH was adjusted to 4, where the chalaza contained a particle size around 1500 nm and held the best capability to stabilize the emulsions. Similarly, the chalaza reaches proper electrical charging (−6 mv) and size (700 nm) after the ionic strength was modified to 0.6 M. Following the characterization of chalaza particles, we successfully generated stable Pickering HIPEs with up to 86% internal phase at proper particle concentrations (0.5–2%). -

Reproduction in Mesozoic Birds and Evolution of the Modern Avian Reproductive Mode Author(S): David J
Reproduction in Mesozoic birds and evolution of the modern avian reproductive mode Author(s): David J. Varricchio and Frankie D. Jackson Source: The Auk, 133(4):654-684. Published By: American Ornithological Society DOI: http://dx.doi.org/10.1642/AUK-15-216.1 URL: http://www.bioone.org/doi/full/10.1642/AUK-15-216.1 BioOne (www.bioone.org) is a nonprofit, online aggregation of core research in the biological, ecological, and environmental sciences. BioOne provides a sustainable online platform for over 170 journals and books published by nonprofit societies, associations, museums, institutions, and presses. Your use of this PDF, the BioOne Web site, and all posted and associated content indicates your acceptance of BioOne’s Terms of Use, available at www.bioone.org/page/terms_of_use. Usage of BioOne content is strictly limited to personal, educational, and non-commercial use. Commercial inquiries or rights and permissions requests should be directed to the individual publisher as copyright holder. BioOne sees sustainable scholarly publishing as an inherently collaborative enterprise connecting authors, nonprofit publishers, academic institutions, research libraries, and research funders in the common goal of maximizing access to critical research. Volume 133, 2016, pp. 654–684 DOI: 10.1642/AUK-15-216.1 REVIEW Reproduction in Mesozoic birds and evolution of the modern avian reproductive mode David J. Varricchio and Frankie D. Jackson Earth Sciences, Montana State University, Bozeman, Montana, USA [email protected], [email protected] Submitted November 16, 2015; Accepted June 2, 2016; Published August 10, 2016 ABSTRACT The reproductive biology of living birds differs dramatically from that of other extant vertebrates. -

School Enrichment Chicken Embryology: an Intracurricular Approach Shaina L. Bennett University of Florida November 2013
School Enrichment Chicken Embryology: An Intracurricular Approach Shaina L. Bennett University of Florida November 2013 1 Introduction Chicken embryology has become a typical program for 4-H youth professionals for school enrichment programs which have become the predominant 4-H delivery mode (Diem, 2001). According to Burrows and Zaremba (1982): One of the original reasons for establishing 4-H as a federally funded youth development program was to give youth extra educational opportunities not realized in rural schools. Today, that philosophy extends to complementing or enriching the curriculum in all schools. (p.18) National 4-H (2006) and others also have documented the disconnection between youth and commercial agricultural production. Chicken embryology is a way to introduce 4-H and agriculture into the classrooms of students that may have never heard about either. Horton, Krieger, and Halasa (2013) also say that elementary school teachers are less likely to enjoy science as well as feel confident about teaching it. Making chicken embryology school enrichment programs more intracurricular has the possibility to increase student knowledge about 4-H and agriculture in addition to easing teachers into teaching more science in the classroom and later gaining confidence in that subject area to teach for the future. Chicken Embryology is a popular 4-H school enrichment program in Florida 4-H. Arnold, Bourdeau, and Nagele (2005) state that 4-H programs are designed to enhance the development of important life skills in youth in a natural, welcoming, positive atmosphere. Therefore, 4-H school enrichment programs should include these aspects. The “Targeting Life Skills Model” is what all programs should be 2 maintaining. -

M. Sc II Semester (Biotechnology) Cells, Molecules and Developmental Biology Unit IV Types of Gametogenesis: Spermatogenesis
M. Sc II Semester (Biotechnology) Cells, Molecules and Developmental Biology Unit IV Types of Gametogenesis: Spermatogenesis and Oogenesis Gametogenesis is the process of formation and differentiation of haploid gametes (sperms and ova) from the diploid primary germ cells, gametogonia (spermatogonia and oogonia) present in primary sex organs called gonads (testes in male and ovaries in female respectively). Gametogenesis is of two types: I. Spermatogenesis and II. Oogenesis. I. Spermatogenesis Definition: It is the formation of haploid, microscopic and functional male gametes, spermatozoa from the diploid reproductive cells, spermatogonia, present in the testes of male organism. Period: In the seasonally breeding animals, the testes undergo testicular cycle in which the testes and their spermatogenic tissue become functional only in the specific breeding season. So in some seasonally breeding mammals like bat, otter and llama, testes enlarge, become functional and descend into the scrotum in the breeding season as become heavier due to accumulation of sperms, while become reduced, non-functional and ascend into the abdomen in other seasons. But in human male, lion, bull, horse etc., the testes lie permanently in the scrotum and spermatogenesis occurs throughout the year. In human male, testes descend into the respective scrotal sacs during seventh month of development under the stimulation of FSH of adenohypophysis. But in some mammals e.g. elephant, echidna, dolphin, whale, seal etc., testes lie permanently in the abdomen (intra-abdominal) mainly due to presence of blubber (thick fatty layer beneath the skin). Spermatogenesis is a continuous process and is completed in about 74 days. Mechanism: Spermatogenesis is divided into two parts: A.