Appendix B Chemical Formulae and Nomenclature
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Transport of Dangerous Goods
ST/SG/AC.10/1/Rev.16 (Vol.I) Recommendations on the TRANSPORT OF DANGEROUS GOODS Model Regulations Volume I Sixteenth revised edition UNITED NATIONS New York and Geneva, 2009 NOTE The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the Secretariat of the United Nations concerning the legal status of any country, territory, city or area, or of its authorities, or concerning the delimitation of its frontiers or boundaries. ST/SG/AC.10/1/Rev.16 (Vol.I) Copyright © United Nations, 2009 All rights reserved. No part of this publication may, for sales purposes, be reproduced, stored in a retrieval system or transmitted in any form or by any means, electronic, electrostatic, magnetic tape, mechanical, photocopying or otherwise, without prior permission in writing from the United Nations. UNITED NATIONS Sales No. E.09.VIII.2 ISBN 978-92-1-139136-7 (complete set of two volumes) ISSN 1014-5753 Volumes I and II not to be sold separately FOREWORD The Recommendations on the Transport of Dangerous Goods are addressed to governments and to the international organizations concerned with safety in the transport of dangerous goods. The first version, prepared by the United Nations Economic and Social Council's Committee of Experts on the Transport of Dangerous Goods, was published in 1956 (ST/ECA/43-E/CN.2/170). In response to developments in technology and the changing needs of users, they have been regularly amended and updated at succeeding sessions of the Committee of Experts pursuant to Resolution 645 G (XXIII) of 26 April 1957 of the Economic and Social Council and subsequent resolutions. -

Nitrogen Oxide - Wikipedia, the Free Encyclopedia Page 1 of 3
Nitrogen oxide - Wikipedia, the free encyclopedia Page 1 of 3 Nitrogen oxide From Wikipedia, the free encyclopedia Nitrogen oxide can refer to a binary compound of oxygen and nitrogen, or a mixture of such compounds: Contents ■ Nitric oxide (NO), nitrogen(II) oxide ■ Nitrogen dioxide (NO2), nitrogen(IV) oxide ■ 1 NOx ■ Nitrous oxide (N2O), nitrogen(I) oxide ■ 2 Derivatives ■ Nitrate radical (NO3), nitrogen(VI) oxide ■ 3 See also ■ Dinitrogen trioxide (N2O3), nitrogen(II,IV) oxide ■ 4 References ■ Dinitrogen tetroxide (N2O4), nitrogen(IV) oxide ■ Dinitrogen pentoxide (N2O5), nitrogen(V) oxide In atmospheric chemistry and air pollution and related fields, nitrogen oxides refers specifically to NOx [1][2] (NO and NO2). Only the first three of these compounds can be isolated at room temperature. N2O3, N2O4, and N2O5 all decompose rapidly at room temperature. Nitrate radical is very reactive. N2O is stable and rather unreactive at room temperature, while NO and NO2 are quite reactive but nevertheless quite stable when isolated. Dinitrogen trioxide, Nitric oxide, NO Nitrogen dioxide, NO2 Nitrous oxide, N2O N2O3 Dinitrogen tetroxide, Dinitrogen pentoxide, N2O4 N2O5 http://en.wikipedia.org/wiki/Nitrogen_oxide 11/2/2010 Nitrogen oxide - Wikipedia, the free encyclopedia Page 2 of 3 NOx Main article: NOx NOx (often written NOx) refers to NO and NO2. They are produced during combustion, especially at high temperature. These two chemicals are important trace species in Earth's atmosphere. In the troposphere, during daylight, NO reacts with partly oxidized organic species (or the peroxy radical) to form NO2, which is then photolyzed by sunlight to reform NO: NO + CH3O2 → NO2 + CH3O NO2 + sunlight → NO + O The oxygen atom formed in the second reaction then goes on to form ozone; this series of reactions is the main source of tropospheric ozone. -

COVALENT COMPOUNDS Unit 2 COVALENT BOND
COVALENT COMPOUNDS Unit 2 COVALENT BOND Forms between two non-metals! Electrons are shared! Co= share Valent = electrons Since electrons are shared, no need to balance charges Covalent bonds form molecules. Molecule - group of neutral atoms held together with a covalent bond Diatomic molecules – molecule consisting of 2 atoms MOLECULES H2O2Br2F2I2N2Cl2 Compound composed of molecules is a molecular compound. MOLECULAR COMPOUNDS Molecular Compounds Properties Gases or liquids Low melting/boiling points Usually made from 2 nonmetals bonded covalently Do not conduct electricity (solid or dissolved) Molecular chemical formula shows how many atoms of each element a molecule contains. H2O – Molecular formula of water 2 Hydrogen atoms, 1 Oxygen atom CO2 – Molecular formula for Carbon Dioxide MOLECULAR 1 Carbon atom, 2 Oxygen atoms FORMULAS H:H - Lewis Dot H-H – Structural Formula H2 – Molecular chemical formula LEWIS STRUCTURES COVALENT BONDING To draw a Lewis Structure, one must know the types of atoms in the molecule, the number of atoms in the molecule and the number of valence electrons for each atom in the molecule. DOUBLE & TRIPLE COVALENT BONDS Sharing a pair of electrons creates a single covalent bond Sharing more than one pair of electrons creates double and triple covalent bonds. 2 shared pairs – Double covalent bond 3 shared pairs – Triple covalent bond Carbon, Nitrogen, Oxygen 1. First element is named first, using the full name of the element 2. The second element is named by dropping ending and adding RULES FOR “–ide” NAMING 3. Prefixes are used to denote # COVALENT of each atom present COMPOUNDS 4. Prefix mono- is never used on the first element 5. -
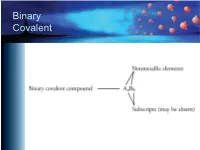
Binary Covalent Common Names
Binary Covalent Common Names – H2O, water – NH3, ammonia – CH4, methane – C2H6, ethane – C3H8, propane – C4H10, butane – C5H12, pentane – C6H14, hexane Naming Binary Covalent CompounDs • If the subscript for the first element is greater than one, inDicate the subscript with a prefix. – We Do not write mono- on the first name. – Leave the "a" off the enD of the prefixes that enD in "a" anD the “o” off of mono- if they are placeD in front of an element that begins with a vowel (oxygen or ioDine). Prefixes mon(o) hex(a) di hept(a) tri oct(a) tetr(a) non(a) pent(a) dec(a) Nitrogen OxiDe Names • N2O3 – name starts with di • N2O5 – name starts with di • NO2 – no initial prefix • NO – no initial prefix Naming Binary Covalent CompounDs • Follow the prefix with the name of the first element in the formula. – N2O3 – dinitrogen – N2O3 – dinitrogen – NO2 – nitrogen – NO – nitrogen Naming Binary Covalent CompounDs • Write a prefix to inDicate the subscript for the seconD element. (Remember to leave the “o” off of mono- anD the “a” off of the prefixes that enD in “a” when they are placeD in front of a name that begins with a vowel.) – N2O3 – dinitrogen tri – N2O5 – dinitrogen pent – NO2 – nitrogen di – NO – nitrogen mon Naming Binary Covalent CompounDs • Write the root of the name of the seconD symbol in the formula. (See the next sliDe.) – N2O3 – dinitrogen triox – N2O5 – dinitrogen pentox – NO2 – nitrogen diox – NO – nitrogen monox Roots of Nonmetals H hydr- F fluor- C carb- Cl chlor- N nitr- Br brom- P phosph- I iod- O ox- S sulf- Se selen- Naming Binary Covalent CompounDs • AdD -ide to the enD of the name. -

Nitrate Formation from Heterogeneous Uptake of Dinitrogen Pentoxide During a Severe Winter Haze in Southern China” by Hui Yun Et Al
Atmos. Chem. Phys. Discuss., https://doi.org/10.5194/acp-2018-698-RC1, 2018 © Author(s) 2018. This work is distributed under the Creative Commons Attribution 4.0 License. Interactive comment on “Nitrate formation from heterogeneous uptake of dinitrogen pentoxide during a severe winter haze in southern China” by Hui Yun et al. Anonymous Referee #1 Received and published: 23 September 2018 The manuscript of Yun et al., reported half month measurement of N2O5, ClNO2 and other relative parameters during heavy haze episodes in Pearl River Delta (PRD) of southern China. The N2O5 uptake coefficient and ClNO2 yield were determined from the observations. The study showed the observation evidence of the enhancement of particulate nitrate in the first several hours can be fully explained by the N2O5 het- erogeneous hydrolysis and even comparable with the nitric acid formed by OH+NO2 during daytime. Overall, the paper contributes to the knowledge of N2O5 heteroge- neous chemistry and highlight the heterogeneous reactions in the formation of partic- ulate nitrate in southern China. The following comments should be addressed before publishing on ACP. C1 Major comments: The steady state assumption to derive the N2O5 uptake coefficient needs to be verified by model simulations under the observed conditions (with input from NO, NO2, O3, VOCs). It is useful to try other method (e.g. Brown et al., 2006) to derive N2O5 uptake coefficient. Brown, S. S., Ryerson, T. B., Wollny, A. G., Brock, C. A., Peltier, R., Sullivan, A. P., Weber, R. J., Dube, W. P., Trainer, M., Meagher, J. F., Fehsenfeld, F. C., and Ravishankara, A. -

Ozone Depletion, Greenhouse Gases, and Climate Change
DOCUMENT RESUME ED 324 229 SE 051 620 TITLE Ozone Depletion, Greenhouse Gaaes, and Climate Change. Proceedings of a Joint Symposium by theBoard on Atmospheric Sciences and Climate andthe Committee on Global Change, National ResearchCouncil (Washington, D.C., March 23, 1988). INSTITUTION National Academy of Sciences - National Research Council, Washington, D.C. SPONS AGENCY National Science Foundation, Washington, D.C. REPORT NO ISBN-0-309-03945-2 PUB DATE 90 NOTE 137p. AVAILABLE FROMNational Academy of Scences, National AcademyPress, 2101 Constitution Avenue, NW, Washington, DC 20418 ($20.00). PUB TYPE Collected Works Conference Proceedings (021) EDRS PRICE MF01 Plus Postage. PC Not Available from EDRS. DESCRIPTORS Air Pollution; *Climate; *Conservation(Environment); Depleted Resources; Earth Science; Ecology; *Environmental Education; *Environmental Influences; Global Approach; *Natural Resources; Science Education; Thermal Environment; World Affairs; World Problems IDENTIFIERS *Global Climate Change ABSTRACT The motivation for the organization of thissymposium was the accumulation of evidence from manysources, both short- and longterm,_that the global climate is in a state of change. Data which defy integrated explanation including temperature, ozone, methane, precipitation and other climate-related trendshave presented troubling problems for atmospheric sciencesince the 1980's. Ten papers from this symposium are presentedhere: (1) "Global Change and the Changing Atmosphere"(William C. Clark); (2) "Stratospheric Ozone Depletion: Global Processes"(Daniel L. Albritton); (3) "Stratospheric Czone Depletion: AntarcticProcesses" (Robert T. Watson); (4) "The Role of Halocarbons in Stratospheric Ozone Depletion" (F. Sherwood Rowland);(5) "Heterogenous Chemical Processes in Ozone Depletion" (Mario J. Molina);(6) "Free Radicals in the Earth's Atmosphere: Measurement andInterpretation" (James G. Anderson); (7) "Theoretical Projections of StratosphericChange Due to Increasing Greenhouse Gases and Changing OzoneConcentrations" (Jerry D. -

2020 Emergency Response Guidebook
2020 A guidebook intended for use by first responders A guidebook intended for use by first responders during the initial phase of a transportation incident during the initial phase of a transportation incident involving hazardous materials/dangerous goods involving hazardous materials/dangerous goods EMERGENCY RESPONSE GUIDEBOOK THIS DOCUMENT SHOULD NOT BE USED TO DETERMINE COMPLIANCE WITH THE HAZARDOUS MATERIALS/ DANGEROUS GOODS REGULATIONS OR 2020 TO CREATE WORKER SAFETY DOCUMENTS EMERGENCY RESPONSE FOR SPECIFIC CHEMICALS GUIDEBOOK NOT FOR SALE This document is intended for distribution free of charge to Public Safety Organizations by the US Department of Transportation and Transport Canada. This copy may not be resold by commercial distributors. https://www.phmsa.dot.gov/hazmat https://www.tc.gc.ca/TDG http://www.sct.gob.mx SHIPPING PAPERS (DOCUMENTS) 24-HOUR EMERGENCY RESPONSE TELEPHONE NUMBERS For the purpose of this guidebook, shipping documents and shipping papers are synonymous. CANADA Shipping papers provide vital information regarding the hazardous materials/dangerous goods to 1. CANUTEC initiate protective actions. A consolidated version of the information found on shipping papers may 1-888-CANUTEC (226-8832) or 613-996-6666 * be found as follows: *666 (STAR 666) cellular (in Canada only) • Road – kept in the cab of a motor vehicle • Rail – kept in possession of a crew member UNITED STATES • Aviation – kept in possession of the pilot or aircraft employees • Marine – kept in a holder on the bridge of a vessel 1. CHEMTREC 1-800-424-9300 Information provided: (in the U.S., Canada and the U.S. Virgin Islands) • 4-digit identification number, UN or NA (go to yellow pages) For calls originating elsewhere: 703-527-3887 * • Proper shipping name (go to blue pages) • Hazard class or division number of material 2. -

Inorganic Nomenclature
Name____________________________ Section____________ Date_______ Inorganic Nomenclature Every compound has its own CHEMICAL FORMULA and its own NAME. The nomenclature (naming systems) for IONIC and MOLECULAR compounds are different. IONIC COMPOUNDS: These consist of any positive ion (except H+) with any negative ion. Note: if H+ is the positive ion, it’s an ACID – see below. + + *The positive ion (CATION) may be a metal ion, e.g., Na , or a polyatomic ion, e.g., NH4 . - 2- *The negative ion (ANION) may be a non-metal ion, e.g., Cl , or a polyatomic ion, such as SO4 Case 1. Representative Metal + Non-metal Compounds Examples: KBr Potassium bromide AlCl3 Aluminum chloride Note: Metal always is first (name unchanged), non-metal is second in formula (name given an –IDE ending). Note: The name does NOT indicate how many of each. EXERCISE: Name the following: NaF _________________ BaS _________________ SrI2 _________________ K2O _________________ Al2O3 ________________ GaN _________________ Give formulas for the following (refer to periodic table only): Cesium phosphide ___________________ Calcium iodide _____________________ Barium fluoride _____________________ Magnesium nitride __________________ Lithium oxide ______________________ Rubidium sulfide ____________________ Strontium bromide __________________ Sodium selenide ____________________ Barium ion ________________________ Nitride ion _________________________ Chloride ion _______________________ Aluminum ion ______________________ - 41 - Case 2. Transition Metal + Non-metal Compounds In general, the ions formed by the transition metals are not predictable. MEMORIZE those given on the last page of handout (Index cards are very helpful). *If the transition metal forms only 1 ion, name the compound as in Case 1. Examples: ZnCl2 Zinc chloride Ag2S Silver sulfide *If the transition metal can form more than one type of ion, name the compounds using Roman numerals to designate the charge on the metal ion. -

Significant Impact of Heterogeneous Reactions of Reactive Chlorine Species on Summertime Atmospheric Ozone and Free-Radical Form
Science of the Total Environment 693 (2019) 133580 Contents lists available at ScienceDirect Science of the Total Environment journal homepage: www.elsevier.com/locate/scitotenv Significant impact of heterogeneous reactions of reactive chlorine species on summertime atmospheric ozone and free-radical formation in north China Xionghui Qiu a,b,QiYingc,⁎, Shuxiao Wang a,b,⁎⁎, Lei Duan a,b, Yuhang Wang d,KedingLue,PengWangf, Jia Xing a,b, Mei Zheng g,MinjiangZhaoa,b, Haotian Zheng a,b, Yuanhang Zhang e,JimingHaoa,b a State Key Joint Laboratory of Environmental Simulation and Pollution Control, School of Environment, Tsinghua University, Beijing 100084, China b State Environmental Protection Key Laboratory of Sources and Control of Air Pollution Complex, Beijing 100084, China c Zachry Department of Civil Engineering, Texas A&M University, College Station, TX, United States d School of Earth and Atmospheric Sciences, Georgia Institute of Technology, Atlanta, GA 30332, United States e State Key Joint Laboratory of Environmental Simulation and Pollution Control, College of Environmental Sciences and Engineering, Peking University,Beijing,China f Department of Civil and Environmental Engineering, The Hong Kong Polytechnic University, 999077, Hong Kong, China g SKL-ESPC and BIC-ESAT, College of Environmental Sciences and Engineering, Peking University, Beijing 100871, China HIGHLIGHTS GRAPHICAL ABSTRACT • This work represents the first high reso- lution regional modeling to quantify the impact of chlorine chemistry on the ox- idation capacity in a polluted urban at- mosphere. • These heterogeneous reactions of reac- tive chlorine species increased the O3, OH, HO2 and RO2 concentrations signifi- cantly for some regions in the Beijing- Tianjin-Hebei (BTH) area. -

Chapter 7: Search for New Fire Suppressant Chemicals
Chapter 7: SEARCH FOR NEW FIRE J. Douglas Mather, Ph.D. SUPPRESSANT CHEMICALS Chemical Development Studies, Inc. Robert E. Tapscott, Ph.D. GlobeTech, Inc. TABLE OF CONTENTS 7.1 Fire Suppressant Replacement Knowledge Prior to the NGP .........................................612 7.1.1 Overview of Early Halon Replacement Efforts ....................................................612 7.1.2 Fire Suppressant Research – 1974 Through 1993.................................................613 7.1.3 DoD Technology Development Plan (1993 to 1997)............................................615 7.1.4 Advanced Agent Working Group (AAWG) .........................................................616 7.1.5 Summary: Alternative Agents and Selection Criteria Prior to the NGP ...............622 7.2 The NGP Approach to New Chemicals Screening..........................................................612 7.3 NGP Surveys of Inorganic Chemical Families................................................................612 7.3.1 Main Group Elements - Group I............................................................................626 7.3.2 Main Group Elements - Group II ..........................................................................627 7.3.3 Main Group Elements - Group III.........................................................................627 7.3.4 Main Group Elements - Group IV.........................................................................628 7.3.5 Main Group Elements - Group V..........................................................................634 -

What Are the Reactive Halogen Gases That Destroy Stratospheric Ozone?
What are the reactive halogen gases that destroy Q7 stratospheric ozone? The chlorine- and bromine-containing gases that enter the stratosphere arise from both human activities and natural processes. When exposed to ultraviolet radiation from the Sun, these halogen source gases are converted to more reactive gases that also contain chlorine and bromine. Some reactive gases act as chemical reservoirs which can then be converted into the most reactive gases, namely ClO and BrO. These most reactive gases participate in catalytic reactions that efficiently destroy ozone. Halogen-containing gases present in the stratosphere can be Reactive halogen gases. The chemical conversion of halogen divided into two groups: halogen source gases and reactive hal- source gases, which involves solar ultraviolet radiation and ogen gases (see Figure Q7-1). The source gases, which include other chemical reactions, produces a number of reactive halo- ozone-depleting substances (ODSs), are emitted at Earth’s gen gases. These reactive gases contain all of the chlorine and surface by natural processes and by human activities (see Q6). bromine atoms originally present in the source gases. The most Once in the stratosphere, the halogen source gases chemically important reactive chlorine- and bromine-containing gases that convert at different rates to form the reactive halogen gases. form in the stratosphere are shown in Figure Q7-1. Throughout The conversion occurs in the stratosphere instead of the tropo- the stratosphere, the most abundant are typically hydrogen sphere for most gases because solar ultraviolet radiation (a com- chloride (HCl) and chlorine nitrate (ClONO2). These two gases ponent of sunlight) is more intense in the stratosphere (see Q2). -

Hydrogen Chloride-Induced Surface Disordering on Ice
Hydrogen chloride-induced surface disordering on ice V. Faye McNeill*†, Thomas Loerting‡§¶, Franz M. Geiger‡§ʈ, Bernhardt L. Trout*, and Mario J. Molina‡§** Departments of ‡Chemistry, §Earth, Atmospheric, and Planetary Sciences, and *Chemical Engineering, Massachusetts Institute of Technology, Cambridge, MA 02139 Contributed by Mario J. Molina, May 4, 2006 Characterizing the interaction of hydrogen chloride (HCl) with gas phase. We find that trace amounts of HCl induce surface polar stratospheric cloud ice particles is essential for understanding change, which we interpret to be formation of a disordered the processes responsible for ozone depletion. We studied the surface region, on ice at a range of conditions that includes interaction of gas-phase HCl with ice between 243 and 186 K by stratospherically relevant temperatures and HCl partial pres- using (i) ellipsometry to monitor the ice surface and (ii) coated-wall sures. We show that the chlorine activation reaction of HCl with flow tube experiments, both with chemical ionization mass spec- ClONO2 on ice is enhanced in the presence of surface disorder, trometry detection of the gas phase. We show that trace amounts as is the uptake of gas-phase CH3COOH. These results impact of HCl induce formation of a disordered region, or quasi-liquid our understanding of the chemistry and physics of ice particles layer, at the ice surface at stratospheric temperatures. We also in the atmosphere. show that surface disordering enhances the chlorine activation reaction of HCl with chlorine nitrate (ClONO2) and also enhances Results acetic acid (CH3COOH) adsorption. These results impact our under- Figs. 1 and 2 show the ellipsometry signals during two studies of standing of the chemistry and physics of ice particles in the ice in the presence of HCl in the gas phase, (i) at constant HCl atmosphere.