Cutis Laxa Precision Panel Overview Indications Clinical Utility
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Tall Stature: a Difficult Diagnosis? Cristina Meazza1* , Chiara Gertosio2, Roberta Giacchero3, Sara Pagani1 and Mauro Bozzola1
Meazza et al. Italian Journal of Pediatrics (2017) 43:66 DOI 10.1186/s13052-017-0385-5 REVIEW Open Access Tall stature: a difficult diagnosis? Cristina Meazza1* , Chiara Gertosio2, Roberta Giacchero3, Sara Pagani1 and Mauro Bozzola1 Abstract Referral for an assessment of tall stature is less common than for short stature. Tall stature is defined as a height more than two standard deviations above the mean for age. The majority of subjects with tall stature show a familial tall stature or a constitutional advance of growth (CAG), which is a diagnosis of exclusion. After a careful physical evaluation, tall subjects may be divided into two groups: tall subjects with normal appearance and tall subjects with abnormal appearance. In the case of normal appearance, the paediatric endocrinologist will have to evaluate the growth rate. If it is normal for age and sex, the subject may be classified as having familial tall stature, CAG or obese subject, while if the growth rate is increased it is essential to evaluate pubertal status and thyroid status. Tall subjects with abnormal appearance and dysmorphisms can be classified into those with proportionate and disproportionate syndromes. A careful physical examination and an evaluation of growth pattern are required before starting further investigations. Physicians should always search for a pathological cause of tall stature, although the majority of children are healthy and they generally do not need treatment to cease growth progression. The most accepted and effective treatment for an excessive height prediction is inducing puberty early and leading to a complete fusion of the epiphyses and achievement of final height, using testosterone in males and oestrogens in females. -

Genetic Investigation of Patients with Tall Stature
2 182 E Vasco de Albuquerque Genetic investigation of tall 182:2 139–147 Clinical Study Albuquerque and others stature patients Genetic investigation of patients with tall stature Edoarda Vasco de Albuquerque Albuquerque1, Mariana Ferreira de Assis Funari2, Elisângela Pereira de Souza Quedas1, Rachel Sayuri Honjo Kawahira3, Raquel Soares Jallad4, Thaís Kataoka Homma1, Regina Matsunaga Martin2,5, Vinicius Nahime Brito2, Alexsandra Christianne Malaquias1,6, Antonio Marcondes Lerario1,7, Carla Rosenberg8, Ana Cristina Victorino Krepischi8, Chong Ae Kim3, Ivo Jorge Prado Arnhold2 and Alexander Augusto de Lima Jorge1 1Unidade de Endocrinologia Genética (LIM25), 2Unidade de Endocrinologia do Desenvolvimento, Laboratório de Hormônios e Genética Molecular (LIM42), Hospital das Clínicas da Faculdade de Medicina, Universidade de São Paulo (USP), São Paulo, Brasil, 3Unidade de Genética do Instituto da Criança, Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo, São Paulo, Brasil, 4Unidade de Neuroendocrinologia, 5Unidade de Doenças Osteometabólicas, Hospital das Clínicas da Faculdade de Medicina, Universidade de São Paulo (USP), São Paulo, Brasil, 6Unidade de Endocrinologia Pediátrica, Correspondence Departamento de Pediatria, Irmandade da Santa Casa de Misericórdia de São Paulo, São Paulo, Brasil, 7Division of should be addressed Metabolism, Endocrinology and Diabetes, Department of Internal Medicine, University of Michigan, Ann Arbor, to A A L Jorge Michigan, USA, and 8Instituto de Biociências (IB), Universidade de São Paulo (USP), São Paulo, Brasil Email [email protected] Abstract Context: Patients with tall stature often remain undiagnosed after clinical investigation and few studies have genetically assessed this group, most of them without a systematic approach. Objective: To assess prospectively a group of individuals with tall stature, with and without syndromic features, and to establish a molecular diagnosis for their growth disorder. -

Megalencephaly and Macrocephaly
277 Megalencephaly and Macrocephaly KellenD.Winden,MD,PhD1 Christopher J. Yuskaitis, MD, PhD1 Annapurna Poduri, MD, MPH2 1 Department of Neurology, Boston Children’s Hospital, Boston, Address for correspondence Annapurna Poduri, Epilepsy Genetics Massachusetts Program, Division of Epilepsy and Clinical Electrophysiology, 2 Epilepsy Genetics Program, Division of Epilepsy and Clinical Department of Neurology, Fegan 9, Boston Children’s Hospital, 300 Electrophysiology, Department of Neurology, Boston Children’s Longwood Avenue, Boston, MA 02115 Hospital, Boston, Massachusetts (e-mail: [email protected]). Semin Neurol 2015;35:277–287. Abstract Megalencephaly is a developmental disorder characterized by brain overgrowth secondary to increased size and/or numbers of neurons and glia. These disorders can be divided into metabolic and developmental categories based on their molecular etiologies. Metabolic megalencephalies are mostly caused by genetic defects in cellular metabolism, whereas developmental megalencephalies have recently been shown to be caused by alterations in signaling pathways that regulate neuronal replication, growth, and migration. These disorders often lead to epilepsy, developmental disabilities, and Keywords behavioral problems; specific disorders have associations with overgrowth or abnor- ► megalencephaly malities in other tissues. The molecular underpinnings of many of these disorders are ► hemimegalencephaly now understood, providing insight into how dysregulation of critical pathways leads to ► -

Level Estimates of Maternal Smoking and Nicotine Replacement Therapy During Pregnancy
Using primary care data to assess population- level estimates of maternal smoking and nicotine replacement therapy during pregnancy Nafeesa Nooruddin Dhalwani BSc MSc Thesis submitted to the University of Nottingham for the degree of Doctor of Philosophy November 2014 ABSTRACT Background: Smoking in pregnancy is the most significant preventable cause of poor health outcomes for women and their babies and, therefore, is a major public health concern. In the UK there is a wide range of interventions and support for pregnant women who want to quit. One of these is nicotine replacement therapy (NRT) which has been widely available for retail purchase and prescribing to pregnant women since 2005. However, measures of NRT prescribing in pregnant women are scarce. These measures are vital to assess its usefulness in smoking cessation during pregnancy at a population level. Furthermore, evidence of NRT safety in pregnancy for the mother and child’s health so far is nebulous, with existing studies being small or using retrospectively reported exposures. Aims and Objectives: The main aim of this work was to assess population- level estimates of maternal smoking and NRT prescribing in pregnancy and the safety of NRT for both the mother and the child in the UK. Currently, the only population-level data on UK maternal smoking are from repeated cross-sectional surveys or routinely collected maternity data during pregnancy or at delivery. These obtain information at one point in time, and there are no population-level data on NRT use available. As a novel approach, therefore, this thesis used the routinely collected primary care data that are currently available for approximately 6% of the UK population and provide longitudinal/prospectively recorded information throughout pregnancy. -

Macrocephaly Information Sheet 6-13-19
Next Generation Sequencing Panel for Macrocephaly Clinical Features: Macrocephaly refers to an abnormally large head, OFC greater than 98th percentile, inclusive of the scalp, cranial bone and intracranial contents. Megalencephaly, brain weight/volume ratio greater than 98th percentile, results from true enlargement of the brain parenchyma [1]. Megalencephaly is typically accompanied by macrocephaly, however macrocephaly can occur in the absence of megalencephaly [2]. Both macrocephaly and megalencephaly can been seen as isolated clinical findings as well as clinical features of a mutli-systemic syndromic diagnosis. Our Macrocephaly Panel includes analysis of the 36 genes listed below. Macrocephaly Sequencing Panel ASXL2 GLI3 MTOR PPP2R5D TCF20 BRWD3 GPC3 NFIA PTEN TBC1D7 CHD4 HEPACAM NFIX RAB39B UPF3B CHD8 HERC1 NONO RIN2 ZBTB20 CUL4B KPTN NSD1 RNF125 DNMT3A MED12 OFD1 RNF135 EED MITF PIGA SEC23B EZH2 MLC1 PPP1CB SETD2 Gene Clinical Features Details ASXL2 Shashi-Pena Shashi et al. (2016) found that six patients with developmental delay, syndrome macrocephaly, and dysmorphic features were found to have de novo truncating variants in ASXL2 [3]. Distinguishing features were macrocephaly, absence of growth retardation, and variability in the degree of intellectual disabilities The phenotype also consisted of prominent eyes, arched eyebrows, hypertelorism, a glabellar nevus flammeus, neonatal feeding difficulties and hypotonia. BRWD3 X-linked intellectual Truncating mutations in the BRWD3 gene have been described in males with disability nonsyndromic intellectual disability and macrocephaly [4]. Other features include a prominent forehead and large cupped ears. CHD4 Sifrim-Hitz-Weiss Weiss et al., 2016, identified five individuals with de novo missense variants in the syndrome CHD4 gene with intellectual disabilities and distinctive facial dysmorphisms [5]. -

Orphanet Report Series Rare Diseases Collection
Marche des Maladies Rares – Alliance Maladies Rares Orphanet Report Series Rare Diseases collection DecemberOctober 2013 2009 List of rare diseases and synonyms Listed in alphabetical order www.orpha.net 20102206 Rare diseases listed in alphabetical order ORPHA ORPHA ORPHA Disease name Disease name Disease name Number Number Number 289157 1-alpha-hydroxylase deficiency 309127 3-hydroxyacyl-CoA dehydrogenase 228384 5q14.3 microdeletion syndrome deficiency 293948 1p21.3 microdeletion syndrome 314655 5q31.3 microdeletion syndrome 939 3-hydroxyisobutyric aciduria 1606 1p36 deletion syndrome 228415 5q35 microduplication syndrome 2616 3M syndrome 250989 1q21.1 microdeletion syndrome 96125 6p subtelomeric deletion syndrome 2616 3-M syndrome 250994 1q21.1 microduplication syndrome 251046 6p22 microdeletion syndrome 293843 3MC syndrome 250999 1q41q42 microdeletion syndrome 96125 6p25 microdeletion syndrome 6 3-methylcrotonylglycinuria 250999 1q41-q42 microdeletion syndrome 99135 6-phosphogluconate dehydrogenase 67046 3-methylglutaconic aciduria type 1 deficiency 238769 1q44 microdeletion syndrome 111 3-methylglutaconic aciduria type 2 13 6-pyruvoyl-tetrahydropterin synthase 976 2,8 dihydroxyadenine urolithiasis deficiency 67047 3-methylglutaconic aciduria type 3 869 2A syndrome 75857 6q terminal deletion 67048 3-methylglutaconic aciduria type 4 79154 2-aminoadipic 2-oxoadipic aciduria 171829 6q16 deletion syndrome 66634 3-methylglutaconic aciduria type 5 19 2-hydroxyglutaric acidemia 251056 6q25 microdeletion syndrome 352328 3-methylglutaconic -
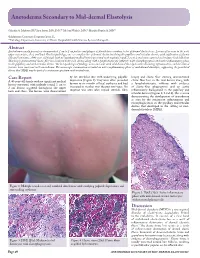
Anetoderma Secondary to Mid-Dermal Elastolysis
Anetoderma Secondary to Mid-dermal Elastolysis Gabriela A. Maloney, BS,* Jane James, MD, PhD,** Michael Welsch, MD,** Marylee Braniecki, MD** *Midwestern University, Downers Grove, IL **Pathology Department, University of Illinois Hospital & Health Sciences System, Chicago, IL Abstract Anetoderma usually presents as circumscribed, 1 cm to 2 cm patches and plaques of flaccid skin secondary to loss of dermal elastic tissue. Lesions often occur in the neck, upper extremities, chest, and back. On histopathology, one sees complete loss of dermal elastin involving the papillary and reticular dermis, with infiltration of plasma cells and histiocytes. A 40-year-old female with no significant medical history presented with multiple round, 1 cm to 2 cm lesions scattered on her upper back and chest. Skin biopsy demonstrated elastic-fiber loss localized to the mid-dermis along with a lymphohistiocytic infiltrate with elastophagocytosis and active inflammatory phase in the papillary and mid-reticular dermis. The histopathological findings were consistent with mid-dermal elastolysis with advancing inflammation, and the clinical features were consistent with anetoderma. The microscopic examination revealed an active inflammatory phase of mid-dermal elastolysis, supporting the postulated theory that MDE may be part of a continuous spectrum with anetoderma. Case Report by lax, wrinkled skin with underlying palpable biopsy and elastic-fiber staining demonstrated A 40-year-old female with no significant medical depression (Figure 1). They were often preceded elastic-fiber loss in the mid-dermis along with history presented with multiple round, 1 cm to by two to six months of local erythema and had a lymphohistiocytic infiltrate with evidence 2 cm lesions scattered throughout the upper increased in number over the past two years. -

Sotos Syndrome
European Journal of Human Genetics (2007) 15, 264–271 & 2007 Nature Publishing Group All rights reserved 1018-4813/07 $30.00 www.nature.com/ejhg PRACTICAL GENETICS In association with Sotos syndrome Sotos syndrome is an autosomal dominant condition characterised by a distinctive facial appearance, learning disability and overgrowth resulting in tall stature and macrocephaly. In 2002, Sotos syndrome was shown to be caused by mutations and deletions of NSD1, which encodes a histone methyltransferase implicated in chromatin regulation. More recently, the NSD1 mutational spectrum has been defined, the phenotype of Sotos syndrome clarified and diagnostic and management guidelines developed. Introduction In brief Sotos syndrome was first described in 1964 by Juan Sotos Sotos syndrome is characterised by a distinctive facial and the major diagnostic criteria of a distinctive facial appearance, learning disability and childhood over- appearance, childhood overgrowth and learning disability growth. were established in 1994 by Cole and Hughes.1,2 In 2002, Sotos syndrome is associated with cardiac anomalies, cloning of the breakpoints of a de novo t(5;8)(q35;q24.1) renal anomalies, seizures and/or scoliosis in B25% of translocation in a child with Sotos syndrome led to the cases and a broad variety of additional features occur discovery that Sotos syndrome is caused by haploinsuffi- less frequently. ciency of the Nuclear receptor Set Domain containing NSD1 abnormalities, such as truncating mutations, protein 1 gene, NSD1.3 Subsequently, extensive analyses of missense mutations in functional domains, partial overgrowth cases have shown that intragenic NSD1 muta- gene deletions and 5q35 microdeletions encompass- tions and 5q35 microdeletions encompassing NSD1 cause ing NSD1, are identifiable in the majority (490%) of 490% of Sotos syndrome cases.4–10 In addition, NSD1 Sotos syndrome cases. -

De Barsy Syndrome
orphananesthesia Anesthesia recommendations for patients suffering from De Barsy syndrome Disease name: De Barsy syndrome ICD 10: Q87.7; OMIM 614438 Synonyms: DBS, De Barsy-Moens-Dierckx syndrome, Progeroid syndrome of De Barsy, Autosomal recessive cutis laxa Type 3 *With 2 gene subdivisions: ARCL3A: caused by a ALDH18A1 mutation ARCL3B: caused by a PYCR1 mutation DeBarsy syndrome is a rare clinical syndrome characterized by cutis laxa, ophthalmic opacification, skeletal malformations, as well as mental and growth retardation. This disease is genetically transmitted in an autosomal recessive fashion. Affected patients often require surgical correction of ophthalmic and orthopaedic abnormalities. This syndrome was first described by A.M. De Barsy in 1967 and less than 100 known cases are documented in the medical literature. Very little has been published on this rare disorder and only a single article has addressed anesthesia case outcomes and management strategies (Aponte, 2010). The diverse collection of clinical manifestations in De Barsy syndrome includes: intra-uterine growth retardation (IUGR), postnatal growth delay, motor delay, cognitive impairment, hypontonia, athetoid movements, malformations, microcephaly, wormian bones, large fontanelles, facial dysmorphism, cataracts, corneal clouding, thin/wrinkled skin, easy bruising, sparse hair, joint laxity, osteopenia, and inguinal hernias. Medicine in progress Perhaps new knowledge Every patient is unique Perhaps the diagnostic is wrong Find more information on the disease, its centres -

Dermatologists Cannot Be Lax About Acquired Cutis Laxa by Warren R
Dermatologists cannot be lax about acquired cutis laxa By Warren R. Heymann, MD Jan. 28, 2019 Acral localized acquired cutis laxa. Loose, redundant skin on the volar finger pads bilaterally that gave them a flat, rounded appearance. Credit: JAADFrom the time I was a medical student, I have always been fascinated by the “little old man” appearance of patient with cutis laxa (CL). In their excellent review, Berk et al note that CL is characterized by abnormal elastic fibers resulting in loose, redundant skin. Typically, skin in CL can easily be pulled and only slowly returns to its original position. Unlike some conditions in the differential diagnosis, notably Ehlers-Danlos syndrome(s) or pseudoxanthoma elasticum, easy bruising or abnormal scarring does not characterize CL. Redundant skin is often most noticeable on the neck, hands, and groin, but can also be seen on the face, creating a premature aging appearance. CL may be inherited or acquired (ACL). Inherited forms include autosomal dominant CL; autosomal recessive CL (ARCL)-I, -IIA, and -IIB; X-linked CL and other variants. Although all of the inherited forms of CL are rare, ARCL has most commonly been reported, particularly ARCL- II. Because of significant overlap among these types, precise clinical classification can be difficult. For the clinical differentiation of the variants and their genetic mutations, please refer to Berk et al (1). The etiology of CL involves abnormalities the elastic fiber network in skin and internal organs. Autosomal dominant CL has been associated with mutations of the elastin gene. X-linked CL is associated with gene mutations ATPA7 and abnormalities of copper transport. -
The Skin in Genetically-Controlled Metabolic Disorders P
Review Article J Med Genet: first published as 10.1136/jmg.10.2.101 on 1 June 1973. Downloaded from Journal of Medical Genetics (1973). 10, 101. The Skin in Genetically-controlled Metabolic Disorders P. C. H. NEWBOLD Department of Medicine, Cambridge University Medical School, Hills Road, Cambridge CB2 2QL Diseased nature oftentimes breaks forth however, it may lead to difficulty in assessing intelli- In strange eruptions.-Henry IV, part 1, III, i. gence, which is within normal range in 50% of The skin is now commonly accepted as a mirror patients. There is suggestive evidence of a re- of internal disease, but as with other looking-glasses, lationship between subnormal folate levels and low the evidence offered may be selected or ignored. intelligence (Carey et al, 1968), and studies have The epidermis is an interesting structure, especially shown increased turnover of folate coenzymes and rich in tyrosine, phenylalanine, tryptophan, and resulting folate depletion in these patients (Carey et histidine, when compared with the corium (Roth- al, 1968; Butterworth, Krumdieck, and Baugh, man, 1965). Tyrosinaemia and histidinaemia do 1971). There is also a high incidence of an organic not include cutaneous manifestations, but culture of brain syndrome following intracranial vascular skin fibroblasts is a helpful diagnostic tool for study- thromboses (Dunn, Perry, and Dolman, 1966), ing metabolic defects such as citrullinaemia, cysti- copyright. nosis, and maple-syrup urine disease (Scriver, 1969). Most of the conditions now to be described are rare, but if these metabolic diseases were com- mon, there could be no human race as we know it. Homocystinuria http://jmg.bmj.com/ This most informative anomaly was discovered during a study of mentally retarded patients in Ire- land (Carson and Neill, 1962). -

RIN2 Deficiency Results in Macrocephaly, Alopecia, Cutis Laxa
REPORT RIN2 Deficiency Results in Macrocephaly, Alopecia, Cutis Laxa, and Scoliosis: MACS Syndrome Lina Basel-Vanagaite,1,2,14 Ofer Sarig,4,14 Dov Hershkovitz,6,7 Dana Fuchs-Telem,2,4 Debora Rapaport,3 Andrea Gat,5 Gila Isman,4 Idit Shirazi,4 Mordechai Shohat,1,2 Claes D. Enk,10 Efrat Birk,2 Ju¨rgen Kohlhase,11 Uta Matysiak-Scholze,11 Idit Maya,1 Carlos Knopf,9 Anette Peffekoven,12 Hans-Christian Hennies,12 Reuven Bergman,8 Mia Horowitz,3 Akemi Ishida-Yamamoto,13 and Eli Sprecher2,4,6,* Inherited disorders of elastic tissue represent a complex and heterogeneous group of diseases, characterized often by sagging skin and occasionally by life-threatening visceral complications. In the present study, we report on an autosomal-recessive disorder that we have termed MACS syndrome (macrocephaly, alopecia, cutis laxa, and scoliosis). The disorder was mapped to chromosome 20p11.21-p11.23, and a homozygous frameshift mutation in RIN2 was found to segregate with the disease phenotype in a large consan- guineous kindred. The mutation identified results in decreased expression of RIN2, a ubiquitously expressed protein that interacts with Rab5 and is involved in the regulation of endocytic trafficking. RIN2 deficiency was found to be associated with paucity of dermal micro- fibrils and deficiency of fibulin-5, which may underlie the abnormal skin phenotype displayed by the patients. Disorders of elastic tissue often share common phenotypic shown to result in decreased secretion of elastin.9,10 In features, including redundant skin, hyperlaxity of joints, addition,