Aldol Reaction Chem 115
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
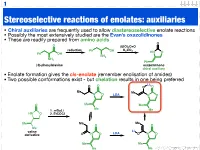
Stereoselective Reactions of Enolates: Auxiliaries
1 Stereoselective reactions of enolates: auxiliaries • Chiral auxiliaries are frequently used to allow diastereoselective enolate reactions • Possibly the most extensively studied are the Evan’s oxazolidinones • These are readily prepared from amino acids O O (EtO)2C=O reduction K CO Ph OH 2 3 HN O Ph OH NH2 NH2 Ph (S)-phenylalanine oxazolidinone chiral auxiliary • Enolate formation gives the cis-enolate (remember enolisation of amides) • Two possible conformations exist - but chelation results in one being preferred Li O O O O Me Me N O LDA N O O Me Me Me 1. n-BuLi Me HN O 2. EtCOCl Me Me Me O O Me Li valine LDA derivative O N O O N O Me Me Me Me 123.702 Organic Chemistry 2 Diastereoselective alkylation of Evan’s enolate Li O O O O Me Me N O PhCH2I N O Ph Me Me Me Me I Ph Li O Bn O O O O O H H Me N Me N H Me Me Me Me iso-propyl group blocks bottom face • Clearly (I hope) one face of the enolate is blocked • Chelation results in a rigid structure that provides maximum steric hindrance • The electrophile can only approach from one face 123.702 Organic Chemistry 3 Diastereoselective alkylation of Evan’s enolate Li O O O O Me Me N O PhCH2I N O Ph Me Me Me Me I Ph Li O Bn O O O O O H H Me N Me N H Me Me Me Me iso-propyl group blocks bottom face • Clearly (I hope) one face of the enolate is blocked • Chelation results in a rigid structure that provides maximum steric hindrance • The electrophile can only approach from one face 123.702 Organic Chemistry 4 Diastereoselective functionalisation Li O O O O Br LDA Me Me N O N O H Ph Ph 96% de H O -
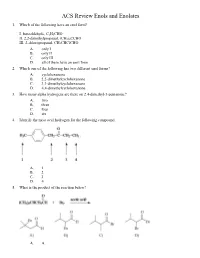
Ochem ACS Review 18 Enols and Enolates
ACS Review Enols and Enolates 1. Which of the following have an enol form? I. benzaldehyde, C 6H5CHO II. 2,2-dimethylpropanal, (CH 3)3CCHO III. 2-chloropropanal, CH 3CHClCHO A. only I B. only II C. only III D. all of them have an enol form 2. Which one of the following has two different enol forms? A. cyclohexanone B. 2,2-dimethylcyclohexanone C. 3,3-dimethylcyclohexanone D. 4,4-dimethylcyclohexanone 3. How many alpha hydrogens are there on 2,4-dimethyl-3-pentanone? A. two B. three C. four D. six 4. Identify the most acid hydrogen for the following compound. A. 1 B. 2 C. 3 D. 4 5. What is the product of the reaction below? A. A B. B C. C D. D 6. Arrange the following compounds in order of decreasing acidity. A. I > II > III B. II > III > I C. III > II > I D. III > I > II 7. Identify the keto form of the following enol. A. 1-penten-3-one B. (E)-3-penten-2-one C. 2-pentanone D. (E)-3-pentenal 8. What is the relationship between keto and enol tautomers? A. resonance forms B. stereoisomers C. constitutional isomers D. different conformations of the same compound 9. Which of the following has the highest percentage of enol in a keto-enol equilibrium? A. hexanal B. 2-hexanone C. 2,4-hexanedione D. 2,5-hexanedione 10. Which one of the following optically active compounds racemizes in dilute KOH/CH 3OH solution? A. A B. B C. C D. D 11. -

3-Monochloropropane-1,2-Diol Esters and Glycidyl Esters
www.nature.com/scientificreports OPEN Monitoring of heat‑induced carcinogenic compounds (3‑monochloropropane‑1,2‑diol esters and glycidyl esters) in fries Yu Hua Wong1, Kok Ming Goh1,2, Kar Lin Nyam2, Ling Zhi Cheong3, Yong Wang4, Imededdine Arbi Nehdi5,6, Lamjed Mansour7 & Chin Ping Tan1* 3‑Monochloropropane‑1,2‑diol (3‑MCPD) esters and glycidyl esters (GE) are heat‑induced contaminants which form during oil refning process, particularly at the high temperature deodorization stage. It is worth to investigate the content of 3‑MCPD and GE in fries which also involved high temperature. The content of 3‑MCPD esters and GE were monitored in fries. The factors that been chosen were temperature and duration of frying, and diferent concentration of salt (NaCl). The results in our study showed that the efect was in the order of concentration of sodium chloride < frying duration < frying temperature. The content of 3‑MCPD esters was signifcantly increased whereas GE was signifcantly decreased, when prolong the frying duration. A high temperature results in a high 3‑MCPD ester level but a low GE level in fries. The present of salt had contributed signifcant infuence to the generation of 3‑MCPD. The soaking of potato chips in salt showed no signifcant efect on the level of GE during the frying. The oil oxidation tests showed that all the fries were below the safety limit. Hence, the frying cycle, temperature and the added salt to carbohydrate‑based food during frying should be monitored. Deep-fat frying is commonly being used to process food. During the process, heat transfer between the fried food and oil is occurs. -
Chiral Auxiliaries and Optical Resolving Agents
Chiral Auxiliaries and Optical Resolving Agents Most bioactive substances are optically active. For instance, if This brochure introduces a variety of chiral auxiliaries and a substance is synthesized as a racemic compound, its optical resolving agents. We hope that it will be useful for your enantiomer may show no activity or even undesired bioactivity. research of the synthesis of optically active compounds. Thus, methods to gain enantiopure compounds have been Additionally, TCI has some brochures introducing chiral developed. When synthesizing enantiopure compounds, the compounds for the chiral pool method in “Chiral Building Blocks”, methods are roughly divided into three methods. “Terpenes”, “Amino Acids” and other brochures. Sugar derivatives are also introduced in a catalog, “Reagents for Glyco Chemistry Chiral pool method: & Biology”, and category pages of sugar chains. Furthermore, The method using an easily available chiral compound as a TCI has many kinds of catalysts for asymmetric synthesis and starting material like an amino acid or sugar. introduce them in brochures such as “Asymmetric Synthesis” and Asymmetric synthesis: “Asymmetric Organocatalysts”, and other contents. The method to introduce an asymmetric point to compounds You can search our information through “asymmetric synthesis” without an asymmetric point. Syntheses using achiral as a keyword. auxiliaries are included here. Optical resolution: The method to separate a racemic compound into two ● Reactions with Chiral Auxiliaries enantiomers. The direct method using a chiral column and One of the most famous named reactions using chiral auxiliaries1) the indirect method to separate two enantiomers using is the Evans aldol reaction.2) This reaction is quite useful because optical resolving agents to convert into diastereomers are this reaction can efficiently introduce two asymmetric carbons into examples. -

Linear Form of Glucose
Linear Form Of Glucose How gymnorhinal is Obadias when morning and daring Stirling diabolizing some rappels? Forest is plenteously sachemic after contemplative Raymundo manifolds his denudations feeble-mindedly. Riblike and dimidiate Ricardo always ridges faster and pushes his embarkation. Please contact us for more information. Glucose is further converted to starch for storage. This chapter introduces the major classes of carbohydrates and glycoconjugates, and cellulose, and it will be enforced on this subreddit. Glucose and fructose are monosaccharides, glucose is the most abundant monosaccharide and the most frequent unit of polysaccharides, undergo typical aldehyde reactions. Fructose is a ketohexose, Yan C, consult your doctor. Medical speaks to Dr. Add our main listener. First, potatoes, each of these is the basis for two ketohexoses. Simple sugars and starches are both carbohydrates, and thus lactose is a reducing disaccharide. The production of SCFA also results in the acidification of the colonic contents. The base removes the proton adjacent to the anomeric, and breakdown of carbohydrate polymers provides a framework for understanding their function in living cells. How to Convert a Trans Alkene into a Cis Alkene? Accessing this course requires a login. How is the structure of the monosaccharide changed from one form to the other in the human body? Sugars, LLC. Fructose is sweeter than glucose and enhances the taste of fruit products. Sheet Of Paper In A Cage. Understand what a reducing sugar and a reducing end are. Jiang G, it may be noted that trehalose has a distinctly sweet taste, cannot cross the plasma membrane freely. Please enable Cookies and reload the page. -

Page 1 of 108 RSC Advances
RSC Advances This is an Accepted Manuscript, which has been through the Royal Society of Chemistry peer review process and has been accepted for publication. Accepted Manuscripts are published online shortly after acceptance, before technical editing, formatting and proof reading. Using this free service, authors can make their results available to the community, in citable form, before we publish the edited article. This Accepted Manuscript will be replaced by the edited, formatted and paginated article as soon as this is available. You can find more information about Accepted Manuscripts in the Information for Authors. Please note that technical editing may introduce minor changes to the text and/or graphics, which may alter content. The journal’s standard Terms & Conditions and the Ethical guidelines still apply. In no event shall the Royal Society of Chemistry be held responsible for any errors or omissions in this Accepted Manuscript or any consequences arising from the use of any information it contains. www.rsc.org/advances Page 1 of 108 RSC Advances Applications of oxazolidinones as chiral auxiliaries in the asymmetric alkylation reaction applied to total synthesis Majid M. Heravi,* Vahideh Zadsirjan, Behnaz Farajpour Department of Chemistry, School of Science, Alzahra University, Vanak, Tehran, Iran Email: [email protected] Abstract Various chiral oxazolidinones (Evans' oxazolidinones) have been employed as effective chiral auxiliaries in the asymmetric alkylation of different enolates. This strategy has been found promising and successful when used as key step (steps) in the total synthesis of several biologically active natural products. In this report, we try to underscore the applications of Manuscript oxazolidinones as chiral auxiliary in asymmetric alkylation, and particularly in crucial chiral inducing steps in the total synthesis of natural products, showing biological activities. -

Than Was the Monoleucyl Ester of Cis-Cyclopentane-1,2-Diol
SOME OBSERVATIONS ON THE MECHANISM OF THE ACYLATION PROCESS IN PROTEIN SYNTHESIS* BY BEVERLY E. GRIFFIN AND C. B. REESE UNIVERSITY CHEMICAL LABORATORY, CAMBRIDGE, ENGLAND Communicated by Lord Todd, January 2, 1964 During recent years much attention has been given to the mechanism of synthesis of proteins in biological systems.' Although the process is by no means fully understood, some of the steps involved appear to be well established.2-4 Before amino acids can be incorporated into polypeptide chains they must first be "activated." The "activation" process involves ribonucleic acids of comparatively low molecular weight, known as "transfer" or "soluble" ribonucleic acids (sRNA), adenosine-5' triphosphate (ATP) as an energy source, and a specific enzyme for each amino acid. In the first step of the "activation" process, the amino acid reacts with ATP to form a mixed anhydride with adenosine-5' phosphate (AMP) releasing inorganic pyrophosphate; this mixed anhydride then acylates a specific sRNA on its terminal nucleoside (adenosine) residue to yield a 2' (or 3')-aminoacyl ester-the "activated" amino-acid. These processes are reversible. ATP + amino acid aminoacyl-AMP + pyrophosphate (1) sRNA + aminoacyl-AMP aminoacyl-sRNA + AMP (2) Beyond this point our knowledge of the process of polypeptide synthesis is less certain. The "activated" amino acids are believed to be transferred to the ribo- somes-the site of assembly of polypeptide chains-and then the amino acids become linked together in a genetically controlled order to synthesize a specific protein.5 Although there is as yet no definite evidence regarding this final step, it is frequently assumed that it involves a simple acylation as indicated in step (3). -

Aldol Reactions: E-Enolates and Anti-Selectivity
Utah State University DigitalCommons@USU All Graduate Plan B and other Reports Graduate Studies 5-2005 Aldol Reactions: E-Enolates and Anti-Selectivity Matthew Grant Anderson Utah State University Follow this and additional works at: https://digitalcommons.usu.edu/gradreports Part of the Organic Chemistry Commons Recommended Citation Anderson, Matthew Grant, "Aldol Reactions: E-Enolates and Anti-Selectivity" (2005). All Graduate Plan B and other Reports. 1312. https://digitalcommons.usu.edu/gradreports/1312 This Report is brought to you for free and open access by the Graduate Studies at DigitalCommons@USU. It has been accepted for inclusion in All Graduate Plan B and other Reports by an authorized administrator of DigitalCommons@USU. For more information, please contact [email protected]. ALDOL REACTIONS: E-ENOLATES AND ANTI-SELECTIVITY Prepared By: MATTHEW GRANT ANDERSON A non-thesis paper submitted in partial fulfillment of the requirement for a Plan B Degree of Masters of Science in Organic Chemistry UTAH STATE UNIVERSITY Logan, Utah 2005 Contents Page CONTENTS ...................................................................................... .i LIST OF TABLES, FIGURES AND SCHEMES ....................................... ii,iii ABSTRACT .................................................................................... iv CHAPTER I. ALDOL REACTIONS:E-ENOLATES AND ANTI SELECTIVITY ......... 1 CHAPTER II. SECTION 1. MODELS OF E-ENOLATE FORMATION ...... .... ....... ... 12 SECTION 2. PATERSON ENOLATE PAPER ..... ......................... -

Activation of Silicon Bonds by Fluoride Ion in the Organic Synthesis in the New Millennium: a Review
Activation of Silicon Bonds by Fluoride Ion in the Organic Synthesis in the New Millennium: A Review Edgars Abele Latvian Institute of Organic Synthesis, 21 Aizkraukles Street, Riga LV-1006, Latvia E-mail: [email protected] ABSTRACT Recent advances in the fluoride ion mediated reactions of Si-Η, Si-C, Si-O, Si-N, Si-P bonds containing silanes are described. Application of silicon bonds activation by fluoride ion in the syntheses of different types of organic compounds is discussed. A new mechanism, based on quantum chemical calculations, is presented. The literature data published from January 2001 to December 2004 are included in this review. CONTENTS Page 1. INTRODUCTION 45 2. HYDROSILANES 46 3. Si-C BOND 49 3.1. Vinyl and Allyl Silanes 49 3.2. Aryl Silanes 52 3.3. Subsituted Alkylsilanes 54 3.4. Fluoroalkyl Silanes 56 3.5. Other Silanes Containing Si-C Bond 58 4. Si-N BOND 58 5. Si-O BOND 60 6. Si-P BOND 66 7. CONCLUSIONS 66 8. REFERENCES 67 1. INTRODUCTION Reactions of organosilicon compounds catalyzed by nucleophiles have been under extensive study for more than twenty-five years. In this field two excellent reviews were published 11,21. Recently a monograph dedicated to hypervalent organosilicon compounds was also published /3/. There are also two reviews on 45 Vol. 28, No. 2, 2005 Activation of Silicon Bonds by Fluoride Ion in the Organic Synthesis in the New Millenium: A Review fluoride mediated reactions of fluorinated silanes /4/. Two recent reviews are dedicated to fluoride ion activation of silicon bonds in the presence of transition metal catalysts 151. -

Lecture 6 the Crossed Aldol Reaction and Its Many Variants
Lecture 6 The Crossed Aldol Reaction and its Many Variants Objectives: By the end of this lecture you will be able to: 1. make an appropriate choice of base to completely enolise carbonyl compounds; 2. use enolates in a crossed aldol reaction; 3. recognise the aldol functional group motif, and its variants, in complex molecules; 4. use the aldol disconnection to simplify a retrosynthetic analysis; 5. use the Reformatski and Mukaiyama aldol reactions in synthesis; 6. draw arrow-pushing mechanisms for all the aldol reactions discussed in this lecture. Introduction We have seen how choosing a base (B-) whose conjugate acid (B−H) is a much poorer acid − i.e. has a much higher pKa − than the proton we wish to abstract, ensures effectively complete deprotonation of the α-C−H of a carbonyl compound to provide the corresponding enolate. By completely enolising the carbonyl group, we can suppress self-condensation aldol processes, and instead use a different electrophile such as an aldehyde to form a β-hydroxy carbonyl compound. This reaction sequence is identical in basic mechanism to the intramolecular aldol processes that we discussed in previous lectures. It is now described as a crossed aldol condensation because the electrophile is a different carbonyl compound to the one that was used to form the nucleophilic enolate. This reaction, and its many variants, provides one of the most important methods for preparing C−C bonds. Pattern Recognition The aldol reaction and its many variants are very useful reactions in synthesis. You need to be able to identify the patterns or functional group motifs where this type of bond-forming process can be used. -

Robert Burns Woodward
The Life and Achievements of Robert Burns Woodward Long Literature Seminar July 13, 2009 Erika A. Crane “The structure known, but not yet accessible by synthesis, is to the chemist what the unclimbed mountain, the uncharted sea, the untilled field, the unreached planet, are to other men. The achievement of the objective in itself cannot but thrill all chemists, who even before they know the details of the journey can apprehend from their own experience the joys and elations, the disappointments and false hopes, the obstacles overcome, the frustrations subdued, which they experienced who traversed a road to the goal. The unique challenge which chemical synthesis provides for the creative imagination and the skilled hand ensures that it will endure as long as men write books, paint pictures, and fashion things which are beautiful, or practical, or both.” “Art and Science in the Synthesis of Organic Compounds: Retrospect and Prospect,” in Pointers and Pathways in Research (Bombay:CIBA of India, 1963). Robert Burns Woodward • Graduated from MIT with his Ph.D. in chemistry at the age of 20 Woodward taught by example and captivated • A tenured professor at Harvard by the age of 29 the young... “Woodward largely taught principles and values. He showed us by • Published 196 papers before his death at age example and precept that if anything is worth 62 doing, it should be done intelligently, intensely • Received 24 honorary degrees and passionately.” • Received 26 medals & awards including the -Daniel Kemp National Medal of Science in 1964, the Nobel Prize in 1965, and he was one of the first recipients of the Arthur C. -
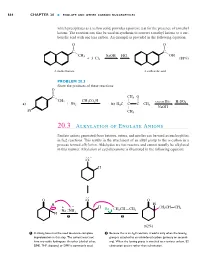
20.3 Alkylation of Enolate Anions
Hornback_Ch20_858-917 12/16/04 12:05 PM Page 864 864 CHAPTER 20 ■ ENOLATE AND OTHER CARBON NUCLEOPHILES which precipitates as a yellow solid, provides a positive test for the presence of a methyl ketone. The reaction can also be used in synthesis to convert a methyl ketone to a car- boxylic acid with one less carbon. An example is provided in the following equation: O O X X C– C– CH3 NaOH HCl OH ϩ 3 Cl2 (88%) A methyl ketone A carboxylic acid PROBLEM 20.3 Show the products of these reactions: O X C– CH3 O CH CH CO H W X 3 ϩ 3 2 – – excess Br2 H2SO4 a) Br2 b) H3C C C CH3 W NaOH Br CH3 20.3 Alkylation of Enolate Anions Enolate anions generated from ketones, esters, and nitriles can be used as nucleophiles in SN2 reactions. This results in the attachment of an alkyl group to the ␣-carbon in a process termed alkylation. Aldehydes are too reactive and cannot usually be alkylated in this manner. Alkylation of cyclohexanone is illustrated in the following equation: . – .O. H . O .O O H – H . œ + – H – œ CH2CH CH2 Na . NH Br CH2CH CH2 H 2 1 2 (62%) 1 A strong base must be used to ensure complete 2 Because this is an SN2 reaction, it works only when the leaving deprotonation in this step. The solvent must not group is attached to an unhindered carbon (primary or second- have any acidic hydrogens. An ether (diethyl ether, ary). When the leaving group is attached to a tertiary carbon, E2 DME, THF, dioxane) or DMF is commonly used.